GENITOURINARY FISTULA
A genitourinary fistula is defined as an abnormal communication between the urinary (ureters, bladder, urethra) and the genital (uterus, cervix, vagina) systems. The true incidence of genitourinary fistula is unknown and varies according to whether the etiology is obstetric or gynecologic. In Asia and Africa, up to 100,000 new cases of obstetric genitourinary fistula are added each year to the estimated pool of 2 million women with unrepaired fistulas (World Health Organization, 2014). For industrialized countries, most fistulas occur iatrogenically from pelvic surgery, and the generally accepted incidence derives from data on surgeries to correct these fistulas. For example, numbers from the National Hospital Discharge Survey of inpatient women show that approximately 4.8 per 100,000 women underwent lower reproductive tract fistula repair (Brown, 2012). This likely is underestimated as many cases are unreported, unrecognized, or treated conservatively. Of genitourinary fistulas, the vesicovaginal fistula is most common and develops significantly more frequently than ureterovaginal fistulas (Goodwin, 1980; Shaw, 2014).
PATHOPHYSIOLOGY
The principles and phases of wound healing aid the understanding of genitourinary fistula pathogenesis. After injury, tissue damage and necrosis stimulate inflammation, and the process of cell regeneration begins (Kumar, 2015). Initially at the injury site, new blood vessels form, that is, angiogenesis. Three to 5 days after injury, fibroblasts proliferate and subsequently synthesize and deposit extracellular matrix, in particular collagen. This fibrosis phase determines the final strength of the healed wound. Collagen deposition peaks approximately 7 days after injury and continues for several weeks. Subsequent scar maturation and organization, termed remodeling, augments wound strength. These phases are interdependent and any disruption of this sequence eventually may create a fistula. Most defects tend to present 1 to 3 weeks after tissue injury. This is a time during which tissues are most vulnerable to alterations in the healing environment, such as hypoxia, ischemia, malnutrition, radiation, and chemotherapy. Edges of the wound eventually epithelialize, and a chronic fistulous tract is thus formed.
CLASSIFICATION
Although many classification systems exist for genitourinary fistula, no single system is considered the accepted standard nor is any one scheme superior in predicting surgical success. Fistulas can develop at any point between the genital and urinary systems, and one classification method reflects the anatomic communication (Table 26-1).
Vesicovaginal fistulas can also be characterized by their size and location in the vagina. They are termed high vaginal, when found proximally in the vagina; low vaginal, when noted distally; or midvaginal, when identified centrally. For instance, posthysterectomy vesicovaginal fistulas are often proximal, or “high” in the vagina, and located at the level of the vaginal cuff.
Others classify vesicovaginal fistula based on the complexity and extent of involvement (Table 26-2) (Elkins, 1999). In this scheme, complicated vesicovaginal fistulas are those that involve pelvic malignancy, prior radiation therapy, a shortened vaginal length, or bladder trigone; those that are distant from the vaginal cuff; or those that measure >3 cm in diameter.
| Simple |
|
| Complicated |
|
In one obstetric classification system, high-risk vesicovaginal fistulas are described by their size (>4 to 5 cm in diameter); involvement of urethra, ureter(s), or rectum; juxtacervical location with an inability to visualize the superior edge; and reformation following a failed repair (Elkins, 1999).
A surgical classification to objectively evaluate obstetric urinary fistula repair has also been introduced (Waaldijk, 1995). In this system, type I fistulas are those that do not involve the urethral closure mechanism, type II fistulas do, and type III fistulas involve the ureter and include other exceptional fistulas. Type II fistulas are divided into: (A) without or (B) with subtotal or total urethra involvement. Type IIB fistulas are further subdivided as: (a) without or (b) with a circumferential configuration around the urethra.
To aid objective comparison of surgical outcomes, a more comprehensive and standardized classification system has been developed (Table 26-3). It integrates fistula distance from the external urethral meatus, fistula size, degree of surrounding tissue fibrosis, and extent of vaginal length reduction (Goh, 2004). This system has good inter- and intraobserver reproducibility and has demonstrated efficacy in predicting which patients are at risk of postfistula urinary incontinence and failure of closure (Goh, 2008, 2009). Despite the availability of these numerous classification systems, most clinicians will, from a practical standpoint, often use the anatomic communication and the relative position in the vagina (high, mid, low) in their initial description of the fistula.
| This new classification divides genitourinary fistulas into four main types, depending on the distance of the fistula’s distal edge from the external urinary meatus. These four types are further subclassified by the size of the fistula, extent of associated scarring, vaginal length, or special considerations. |
| Type 1: Distal edge of fistula >3.5 cm from external urinary meatus |
| Type 2: Distal edge of fistula 2.5–3.5 cm from external urinary meatus |
| Type 3: Distal edge of fistula 1.5 to <2.5 cm from external urinary meatus |
| Type 4: Distal edge of fistula <1.5 cm from external urinary meatus |
|
|
ETIOLOGY
Congenital genitourinary fistulas are rare, but if found, are commonly associated with other renal or urogenital abnormalities. Thus, most vesicovaginal fistulas are acquired and typically result from either obstetric trauma or pelvic surgery.
In developing countries, more than 70 percent of genitourinary fistulas arise from obstetric trauma, specifically from prolonged or obstructed labor or complicated cesarean delivery (Arrowsmith, 1996; Kumar, 2009; Raassen, 2014). Their development in this setting often reflects social practices or obstetric management common to a particular community or geographic region. For example, both childbearing at a young age, before the pelvis has completely developed or fully grown, and female circumcision, more correctly termed female genital mutilation, may significantly narrow the vaginal introitus and obstruct labor. Prolonged obstructed labor or anatomic malpresentation of the presenting fetal part can cause pressure and ischemic necrosis of the anterior vaginal wall and bladder, subsequently resulting in fistula formation. Alternatively, the vagina may be damaged by instruments used to deliver stillborn infants or perform abortion. Malnutrition and limited health care in many of these countries can further diminish wound healing.
In contrast, in most developed countries, fistulas uncommonly follow obstetric procedures or deliveries. Rarely, cesarean deliveries, usually those accompanied by obstetric complications, have led to complex urinary fistula (Billmeyer, 2001). Similarly, rare cases following cervical cerclage have been reported (Massengill, 2012).
In developed countries, iatrogenic injury during pelvic surgery is responsible for 90 percent of vesicovaginal fistulas, and the accepted incidence of fistula formation after pelvic surgery is 0.1 to 2 percent (Harris, 1995; Hilton, 2012a,b; Tancer, 1992). The remaining fistulas result from procedures performed by urologists and by colorectal, vascular, and general surgeons. In industrialized countries, hysterectomy is the most common surgical precursor to vesicovaginal fistula, accounting for approximately 75 percent of fistula cases (Symmonds, 1984). When all hysterectomy types are considered, vesicovaginal fistula is estimated to complicate 0.8 per 1000 procedures (Harkki-Siren, 1998). In their review of more than 62,000 hysterectomy cases, laparoscopic hysterectomies were associated with the greatest incidence (2 per 1000), followed by abdominal (1 per 1000), vaginal (0.2 per 1000), and supracervical (0 per 1000) hysterectomies. With hysterectomy for benign disease, Duong and colleagues (2009) noted that bladder wall laceration extending into the bladder neck or a ureteral orifice (trigone) significantly increased the risk of subsequent vesicovaginal fistula.
Because most genitourinary fistulas follow pelvic surgery, prevention and intraoperative recognition of lower urinary tract injury is imperative. As discussed extensively in Chapter 40, intraoperative cystoscopy has been shown to improve the detection rate of lower urinary tract injuries. This in turn may ultimately translate into a lower incidence of genitourinary fistula. Thus, intraoperative cystoscopy can be a useful adjunct, particularly in cases in which the ureters or bladder are suspected to have been at increased injury risk.
Other etiologies for urinary tract fistulas include radiation therapy, malignancy, trauma, foreign bodies, infections, pelvic inflammation, and inflammatory bowel disease. Of these, radiation therapy induces an endarteritis that can lead to tissue necrosis and subsequent potential fistula formation. This modality is a frequent cause, and some series have reported that up to 6 percent of genitourinary fistulas can result from radiation (Lee, 1988). Although most damage following this therapy develops within weeks and months, associated fistulas have been reported to present up to 20 years after the original insult (Graham, 1967; Zoubek, 1989).
Malignancy is commonly linked with tissue necrosis and may lead to urinary fistula formation. Emmert and Kohler (1996) found a 1.8-percent incidence of rectovaginal and vesicovaginal fistula in their analysis of nearly 2100 women with cervical cancer. Thus, tissue biopsy is routinely considered during diagnostic evaluation of women with a fistula and history of malignancy.
Trauma sustained during sexual activity or sexual assault can result in genitourinary fistula formation and has been estimated to precede 4 percent of these defects (Kallol, 2002; Lee, 1988). Foreign bodies such as a neglected pessary or vesical calculi are also documented causes (Arias, 2008; Dalela, 2003). Given that transurethral catheter placement has been linked to urethrovaginal fistula, this commonly used device should be placed, maintained, and removed with care (Dakhil, 2014). Foreign material introduced during surgery such as collagen injected transurethrally and complications resulting from synthetic mesh placement for urinary incontinence or pelvic organ prolapse are other inciting agents (Blaivas, 2014; Firoozi, 2012; Pruthi, 2000). Also, during sling surgeries, excess sling tension may increase tissue stress and necrosis. Thus, initial material selection and patient evaluation for poor wound healing risk factors are important prevention steps (Giles, 2005). Ideally, the material selected minimizes the normal foreign-body reaction, is nontoxic and nonantigenic, and is porous enough to admit immune and phagocytic cells and promote native tissue ingrowth (Birch, 2002). Mesh selection is further discussed in Chapter 24.
Other rare causes of fistula formation include infections such as lymphogranuloma venereum, urinary tuberculosis, pelvic inflammation, and syphilis; inflammatory bowel disease; and autoimmune disease (Ba-Thike, 1992; Monteiro, 1995). Additionally, conditions that interfere with healing such as poorly controlled diabetes mellitus, smoking, local infection, peripheral vascular disease, and chronic corticosteroid use are potential risks.
SYMPTOMS
Vesicovaginal fistula classically presents with unexplained continuous urinary leakage from the vagina after a recent operation. Depending on the size and location of the fistula, the urine amount will vary. Occasionally small-volume, intermittent leakage is mistaken for postoperative stress incontinence. For this reason, patients with new-onset urinary leakage, particularly in the setting of recent pelvic surgery, are examined thoroughly to exclude fistula formation. Other less specific symptoms of genitourinary fistula include fever, pain, ileus, and bladder irritability.
Vesicovaginal fistula may present days to weeks after the initial inciting surgery, and those following hysterectomy typically present at 1 to 3 weeks. Some fistulas, however, have longer latency, and symptoms may develop several years later.
DIAGNOSIS
A thorough history and physical examination identifies most cases of vesicovaginal fistula. Accordingly, information is documented regarding obstetric deliveries, prior surgeries, previous fistula management, and malignancy treatment, especially pelvic surgery and radiation therapy.
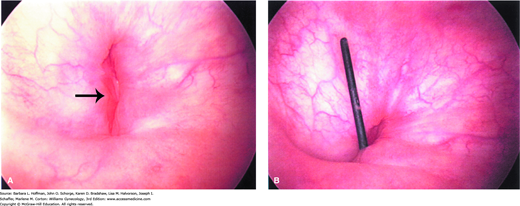
Physical examination is equally informative, and vaginal inspection often will identify the defect. A meticulous assessment for other fistulous tracts is performed, and their location and size noted. Visual assistance with an endoscopic lens and translucent vaginal speculum can sometimes help identify a vaginal-apex fistula, which can be more difficult to detect.
It is essential to differentiate between “extraurethral” urinary leakage, as with a fistula, and “transurethral” leakage, that is, through the urethra, as with stress urinary incontinence. Occasionally, the vaginal fluid source is unclear, and a small amount of urine can easily be mistaken for vaginal discharge. Measurement of the vaginal fluid’s creatinine content can sometimes be used to confirm its origin. Although creatinine levels in urine vary, with mean levels reaching 113.5 mg/dL, a value >17 mg/dL is consistent with urine (Barr, 2005). In contrast, fluid with a concentration <5 mg/dL is highly unlikely to be human urine.
Although a genitourinary fistula ideally is visualized directly, inspection at times is unrevealing. In these circumstances, retrograde bladder instillation of visually distinct solutions such as sterile milk or dilute methylene blue or indigo carmine can often indicate a fistula and aid in its localization.
If the presence of a urinary fistula is uncertain or its vaginal location is not identified, a three-swab test, commonly known as the “tampon test,” is recommended (Moir, 1973). This test is commonly performed with a tampon. However, we recommend using two to four pieces of gauze sequentially packed into the vaginal canal. A diluted solution of methylene blue or indigo carmine is instilled into the bladder using a transurethral catheter. Notably, use of the former may increase given current indigo carmine shortages. After 15 to 30 minutes of routine activity, the gauze is removed serially from the vagina, and each is inspected for dye. The specific gauze colored with dye suggests the fistula location—a proximal or high location in the vagina for the innermost gauze and a low or distal fistula for the outermost. If the distally placed sponge is stained with dye, however, confirmation that it was not contaminated by urine leaking out through the urethra, as in the case of stress urinary incontinence, is essential.
Cystourethroscopy is another valuable diagnostic tool (Fig. 26-1). It permits fistula localization, determination of its proximity to the ureteral orifices, inspection for multiple fistula sites, and assessment of surrounding bladder mucosa viability. In addition, the use of cystourethroscopy and vaginoscopy concurrently to identify vesicovaginal fistula has been described (Andreoni, 2003).
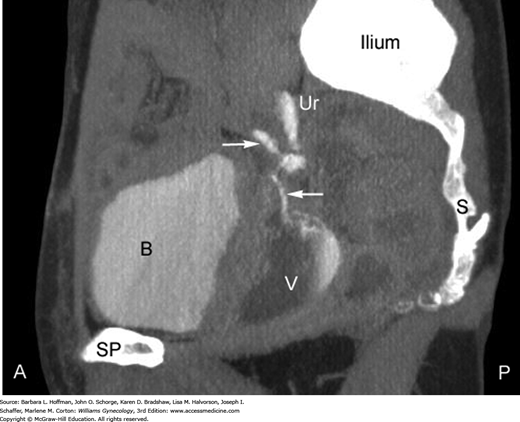
Concomitant ureteral involvement is estimated to complicate 10 to 15 percent of vesicovaginal fistula cases and is sought during diagnostic evaluation (Goodwin, 1980). At our institution, intravenous contrast-enhanced computed tomography (CT) scanning in the excretory phase has become the preferred diagnostic test after initial cystourethroscopic survey is completed (Fig. 26-2). Selection of modalities other than CT for fistulous tract identification may be considered based on cost or availability. First, intravenous pyelography (IVP) can adequately confirm integrity of the upper collecting system and exclude ureteral involvement in a fistula. Second, retrograde pyelography may be used. Often carried out in conjunction with cystoscopy, it is performed by placing a small catheter into the distal ureter. Contrast material is injected through the catheter into one or both ureters. Fluoroscopic or conventional radiographs are then obtained. Retrograde pyelography generally has been reported to have the same diagnostic value as IVP.
In some instances resources are scarce, cost may be a limitation, and access to specialized diagnostic imaging is a challenge. With some advance planning, phenazopyridine hydrochloride (Pyridium) can be used in conjunction with the three-swab test to determine ureteral involvement, as a very rudimentary alternative to the aforementioned more sophisticated imaging. This tablet is administered orally, is excreted renally, acts as a topical bladder analgesic, and stains urine orange as a side effect. Women with suspected ureteral involvement are instructed to take a 200-mg dose a few hours before their clinic appointment. The steps of the three-swab test are then performed, as described earlier. In this case, if the most proximal (innermost) gauze is colored with orange dye, ureteral involvement is suspected. If both orange and blue dyes are seen, then involvement of both the bladder and ureter(s) is suspected.
Voiding cystourethrography (VCUG) can help confirm the presence, location, and number of fistulous tracts. In this, the bladder is filled via catheter with contrast dye, and fluoroscopic images of the lower urinary tract are obtained during patient micturition. However, CT has largely replaced this modality. Transabdominal sonography with applied color Doppler to identify flow through the fistula has been suggested as another diagnostic option (Volkmer, 2000). However, without color Doppler, sonography failed to identify 29 percent of vesicovaginal fistula cases in one study (Adetiloye, 2000).
TREATMENT
Occasionally, genitourinary fistulas may spontaneously close during continuous bladder drainage using an indwelling urinary catheter. Approximately 12 percent of women treated by sustained catheterization alone had fistulas that healed spontaneously (Oakley, 2014; Waaldijk, 1994). Romics and colleagues (2002) found that in 10 percent of cases, urinary fistulas close spontaneously after 2 to 8 weeks of transurethral catheterization, especially if the fistula is small (2- to 3-mm diameter). Another series reported fistulas up to 2 cm in diameter spontaneously healed in 50 to 60 percent of patients treated with an indwelling catheter (Waaldijk, 1989).
Despite these series, data that correlate fistula size and success of conservative management are limited. Many reports of successful spontaneous closure with catheter drainage have been limited to fistulas that were 1 cm in size or smaller (Lentz, 2005; Ou, 2004). Many studies are vague regarding how fistula size is measured, and each series has potential for considerable bias in its selection criteria. However, in general, the larger a fistula, the less likely it is to heal without surgery.
Evidence regarding the duration of catheter drainage also varies. Regardless, many agree that if a fistula has not closed within 4 weeks, it is unlikely to do so. This may be secondary to epithelialization of the fistulous tract (Davits, 1991; Tancer, 1992). Moreover, continued urinary drainage may lead to further bladder inflammation and irritation (Zimmern, 1991
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree