INTRODUCTION
Infertility is defined as the inability to conceive after 1 year of unprotected intercourse of reasonable frequency. It can be subdivided into primary infertility, that is, no prior pregnancies, and secondary infertility, referring to infertility following at least one prior conception.
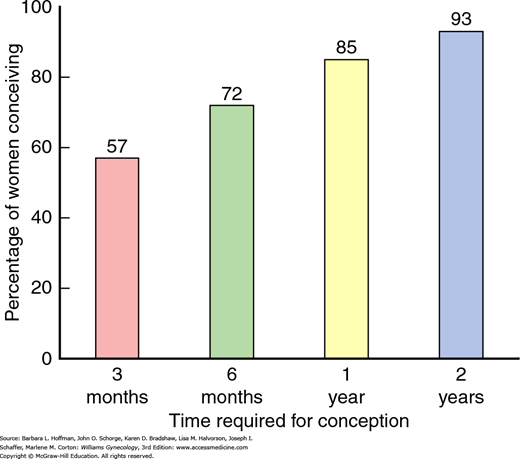
Conversely, fecundability is the ability to conceive, and data from large population studies show that the monthly probability of conceiving is 20 to 25 percent. In those attempting conception, approximately 50 percent of women will be pregnant at 3 months, 75 percent will be pregnant at 6 months, and more than 85 percent will be pregnant by 1 year (Fig. 19-1) (Guttmacher, 1956; Mosher, 1991).
Infertility is common and affects 10 to 15 percent of reproductive-aged couples. Of note, even without treatment, approximately half of women will conceive in the second year of attempting. According to the National Survey of Family Growth, the percentage of married women who reported infertility fell from 8.5 percent in 1982 to 6.0 percent in 2006 to 2010. In comparison, the percentage of women aged 15 to 44 years who had ever used infertility services increased from 9 percent in 1982 to 12 percent in 2002, with a peak of 15 percent in 1995 (Chandra, 2013, 2014). Interpretation of these data is complicated by ongoing changes in marriage rates, intentional delays in childbearing, and socioeconomic and educational status changes in a growing immigrant community. Nevertheless, well-publicized successes in infertility treatment now give patients greater hope that medical intervention will help them achieve their goal.
Most couples are more correctly considered to be subfertile, rather than infertile, as they will ultimately conceive if given enough time. This concept of subfertility can be reassuring to couples. However, there are obvious exceptions, such as the woman with bilaterally obstructed fallopian tubes or the azoospermic male. In general, infertility evaluation is for any couple that has failed to conceive in 1 year. But, several scenarios may prompt earlier intervention. For example, to delay assessment in an anovulatory woman or a woman with a history of severe pelvic inflammatory disease (PID) may not be appropriate. Of particular note, fecundability is highly age-related, with a significant decrease beginning at approximately 32 years of age and more rapid decline after age 37 (American Society for Reproductive Medicine, 2014a). This decline in conception rates is associated with an increase in poor pregnancy outcomes, primarily due to increased aneuploidy rates. Thus, most experts agree that evaluation is considered after only 6 months in women older than 35 years.
Prior to initiating infertility treatment, a patient’s health status must be optimized for an anticipated pregnancy. Ideally, these issues are addressed prior to referral to an infertility specialist whenever possible. Topics include appropriate vaccination; screening for diabetes, infectious diseases, or genetic disorders; folic acid supplementation; weight reduction; and cessation of cigarette smoking or illicit drug use. Additional information is provided later in this chapter and in Table 1-17.
ETIOLOGY OF INFERTILITY
Successful pregnancy requires a complex sequence that includes ovulation, ovum pick-up by a fallopian tube, fertilization, transport of a fertilized ovum into the uterus, and implantation into a receptive uterine cavity. In the male system, sperm of adequate number and quality must be deposited at the cervix near the time of ovulation. Remembering these critical events can aid in developing an appropriate evaluation and treatment strategy.
In general, infertility can be attributed to the female partner one third of the time, the male partner one third of the time, and both partners in the remaining one third. This approximation emphasizes the value of assessing both partners before instituting therapy. Although a complete investigation may not be required before instituting therapy if a clear etiology is present, strong consideration is given to finishing testing if pregnancy is not rapidly achieved. Estimates of the incidence of various causes of infertility are shown in Table 19-1 (Abma, 1997; American Society for Reproductive Medicine, 2006).
Both partners are urged to attend the initial consultation. This time provides an excellent opportunity to educate regarding the normal conception process and methods to optimize their natural fertility. Such efforts may obviate the need for expensive and time-consuming interventions (American Society for Reproductive Medicine, 2013a). Couples are informed of the concept of a fertile window for conception. The chance of conception is increased from the 5 days preceding ovulation through the day of ovulation (Wilcox, 1995). If the male partner has normal semen characteristics, a couple ideally has daily intercourse during this period to maximize the chance of conception. Although sperm concentrations will drop with increasing coital frequency, this decrease is generally too small to significantly lower the chance of fertilization (Stanford, 2002). Couples are also reminded to avoid oil-based lubricants, which are harmful to sperm. Many myths surround the ability to conceive. Examples, such as the importance of coital position and the need to remain horizontal following ejaculation, can add undue stress to an already stressful situation and should be dispelled.
MEDICAL HISTORY
As with any medical condition, a thorough history and physical examination is critical (American Society for Reproductive Medicine, 2012a). Specifically, questions cover menstruation (frequency, duration, recent change in interval or duration, hot flushes, dysmenorrhea), prior contraceptive use, coital frequency, and infertility duration. Previous endometriosis, recurrent ovarian cysts, leiomyomas, sexually transmitted diseases, or PID is also pertinent. Because prior conception indicates ovulation and a patent fallopian tube in the patient’s past, this history is sought. A prolonged time to conception may suggest borderline fertility and may increase the chance of determining an etiology. Pregnancy complications such as miscarriage, preterm delivery, retained placenta, postpartum dilatation and curettage, chorioamnionitis, or fetal anomalies are also recorded. Prior abnormal Pap testing may be relevant, particularly if a woman underwent cervical conization, which can diminish cervical mucus and cervical competence. A coital history, including frequency and timing of intercourse, is also obtained. Symptoms such as dyspareunia may point to endometriosis and a need for earlier diagnostic laparoscopy for the female partner.
During medical history inventory, symptoms of hyperprolactinemia or thyroid disease are sought. Symptoms of androgen excess such as acne or hirsutism may point to polycystic ovarian syndrome (PCOS) or much less commonly, congenital adrenal hyperplasia. Prior chemotherapy or pelvic irradiation may suggest ovarian failure. This is also an excellent opportunity to ensure that all indicated vaccinations are current, as several are contraindicated once pregnancy is achieved (American Society for Reproductive Medicine, 2013d). Vaccine indications and schedules are found in Table 1-2.
Questions regarding medications include over-the-counter agents, such as nonsteroidal antiinflammatory drugs, that may adversely affect ovulation. In most instances, herbal remedies are discouraged. Women are encouraged to take a daily vitamin with at least 400 μg of folic acid to decrease the chance of neural-tube defects. In those with a previously affected child, 4 g is taken orally daily (American College of Obstetricians and Gynecologists, 2014b).
Previous pelvic and abdominal surgeries, especially if linked to endometriosis or adhesion formation, can lower fertility. As examples, operations for ruptured appendicitis or diverticulitis raise suspicion for pelvic adhesive disease or tubal obstruction or both. Prior uterine surgery can predispose to pain, bowel obstruction, or extra- or intrauterine adhesions with resultant infertility. When planning surgery, reducing adhesion formation is a priority, and meticulous surgical technique and minimally invasive surgical approaches are favored. Surgical adhesion barriers, described in Chapter 11, lower postoperative adhesion rates. However, no strong evidence exists that their use improves fertility, decreases pain, or lowers bowel obstruction rates (American Society for Reproductive Medicine, 2013b).
A social history focuses on lifestyle factors such as eating habits. Abnormalities in gonadotropin-releasing hormone (GnRH) and gonadotropin secretion are clearly related to body mass indices >25 or <17 (Grodstein, 1994a). An estimated 30 to 50 percent of women, depending on race and ethnicity, are overweight or obese. Most agree that this incidence is increasing (American Society for Reproductive Medicine, 2008c; Hedley, 2004). In these women, infertility is primarily related to an increased incidence of ovulatory dysfunction, but data also suggest that fecundity is lower among ovulatory obese women. Although difficult to achieve, even modest weight reduction in overweight women is correlated with normalized menstrual cycles and subsequent pregnancies (Table 19-2).
| Factor | Impact on Fertility |
| Obesity (BMI >35) | 2-fold increase TTC |
| Underweight (BMI <19) | 4-fold increase TTC |
| Smoking | 1.6-fold increase RR |
| Alcohol (>2/day) | 1.6-fold increase RR |
| Illicit drugs | 1.7-fold increase RR |
| Toxins | 1.4-fold increase RR |
| Caffeine (>250 mg/day) | 45% decrease fecundability |
Accumulating data also suggest that cigarette smoking lowers fertility rates (American Society for Reproductive Medicine, 2012d). At least one fifth of reproductive-aged men and women in the United States smoke cigarettes (Centers for Disease Control and Prevention, 2014). The prevalence of infertility is higher, and the time to conception is longer in women who smoke, or even those exposed passively to cigarette smoke. Moreover, smoking’s negative effects on female fecundity do not appear to be overcome by assisted reproductive technologies (ART). A 5-year prospective study of 221 couples found that the risk of failing to conceive with ART was more than doubled in smokers. Each year that a woman smoked was associated with a 9-percent increase in the risk of unsuccessful ART cycles (Klonoff-Cohen, 2001).
Toxins in the smoke can accelerate follicular depletion and increase genetic mutations in gametes or early embryos (Zenzes, 2000). Smoking is associated with an increased miscarriage rate in both natural and assisted conception cycles. The mechanism for this is unclear, but the vasoconstrictive and antimetabolic properties of some cigarette smoke components such as nicotine, carbon dioxide, and cyanide may lead to placental insufficiency. Specifically, smoking has been linked to higher rates of abruption, fetal growth restriction, and preterm labor (Cunningham, 2014). In addition, smoking in pregnant women is associated with an increased risk of trisomy 21 that results from maternal meiotic nondisjunction (Yang, 1999). Admittedly, current data do not prove causation, but only correlation, between smoking and infertility or adverse pregnancy outcomes.
The effect of smoking on male fertility is more difficult to discern. Although smokers often have comparatively reduced sperm concentrations and motility, these often remain within the normal range.
Smoking is discouraged for both male and female partners planning pregnancy. The desire for pregnancy can be a powerful motivator toward cessation (Augood, 1998). Education is the most important first step (Table 19-3). If behavioral approaches fail, use of medical adjuncts such as nicotine replacement therapy, bupropion (Zyban), or varenicline (Chantix) may prove effective (Table 1-4). Nicotine preparations are designated as category D. Bupropion and varenicline are non-nicotine Food and Drug Administration (FDA)-approved agents and carry a category C designation (Fiore, 2008). Ideally pharmacological smoking cessation therapies are best used prior to conception.
Alcohol consumption also should be limited. Heavy alcohol intake decreases fertility in women, and in men has been associated with a decrease in sperm counts and increase in sexual dysfunction (Klonoff-Cohen, 2003; Nagy, 1986). A standardized alcoholic drink is typically defined as 12 ounces of beer, 5 ounces of wine, or 1.5 ounces of hard alcohol. Based on several studies, five to eight drinks per week negatively affects female fertility (Grodstein, 1994b; Tolstrup, 2003). As alcohol is also detrimental to early pregnancy, it is prudent to advise patients to avoid excessive alcohol consumption while trying to conceive.
Caffeine is one of the most widely used pharmacologically active substances in the world. Studies evaluating a potential relationship between caffeine and impaired fertility have varied in design and resulted in conflicting findings. One large prospective trial found no association between either total caffeine intake or coffee consumption and fecundability (Hatch, 2012). Despite this, recommendations of caffeine intake moderation in infertile women seem prudent.
Illicit drugs may also affect fecundability. Marijuana suppresses the hypothalamic-pituitary-gonadal axis in both men and women, and cocaine can impair spermatogenesis (Bracken, 1990; Smith, 1987).
Increasing information suggests that some male and female infertility may result from environmental contaminants or toxins (Giudice, 2006). Endocrine-disrupting chemicals (EDCs) have been shown to be reproductive toxicants. Examples are dioxins and polychlorinated biphenyls, as well as agricultural pesticides and herbicides, phthalates (used in making plastic materials), lead, and bisphenol A (used in the manufacture of polycarbonate plastic and resins) (Hauser, 2008; Mendola, 2008). EDC exposure is implicated to underlie a broad range of women’s reproductive disorders. Lower fecundability and lower birthweight show the most solid evidence for this correlation (Caserta, 2011). Although direct links to infertility in humans are not conclusive, clinicians should counsel patients that environmental exposures to toxic substances should be avoided if possible. Currently, these cautions should be discussed carefully to avoid alarm.
The ethnic background and family history of both partners influences the need for preconceptional testing. A family history of infertility, recurrent miscarriage, or fetal anomalies may point to a genetic etiology. Although the inheritance pattern is complex, data suggest that both PCOS and endometriosis occur in familial clusters. For example, a woman carries an estimated sevenfold increased risk of endometriosis over that of the general population if a single first-degree family member has the disease (Moen, 1993).
Genetic carrier screening can be offered preconceptionally or following conception. Testing before conception is often more straightforward and less stressful for the couple than delaying until pregnancy has been achieved. However, insurance carriers may decline to reimburse for this evaluation (American Academy of Pediatrics and American College of Obstetricians and Gynecologists, 2012). Preconception carrier screening also allows a couple to consider the most complete range of reproductive options. Knowing the risk of having an affected child, a couple may consider preimplantation genetic diagnosis, prenatal genetic testing, or the use of donor gametes (American College of Obstetricians and Gynecologists, 2014e). In the absence of known family history of genetic disease, it is reasonable to offer genetic carrier screening to the woman first and test the male partner only if the mother has positive results.
Specific recommendations for genetic carrier screening have been published by the American College of Obstetricians and Gynecologists (2009, 2014a,c,d), by the American College of Medical Genetics and Genomics, and by other advocacy groups and societies (Grody, 2013; Gross, 2013; Pletcher, 2006). These opinions have changed over time and continue to vary across organizations. No doubt, screening guidelines will continue to evolve as technology advances and the costs and benefits of obtaining this information become more evident. Certain disorders are more common in specific ethnic groups, although it is essential to note that there are no disorders found uniquely in a certain ethnic or racial group. Many families may be interracial and ethnic background may be unknown. For example, cystic fibrosis screening was initially recommended only for the non-Hispanic white population and those of Ashkenazi Jewish descent. However, this recommendation now extends to all individuals to account for increased numbers of individuals with mixed ethnicity and inaccuracies based on personal reporting (Ross, 2011; Tanner, 2014).
Traditional genotyping methods detect a limited number of mutations, and these tests have been developed to be specific for the more common mutations found in the ethnic group most at risk. Expanded genotyping panels have been developed but are costly and remain limited. More recently, the cost of DNA sequencing has been greatly reduced due to the emergence of next-generation sequencing (NGS) techniques (Hallam, 2014). This allows rapid and efficient testing of many genes and thousands of mutations concurrently. Panethnic population screening by NGS for numerous genetic disorders is now technically feasible. Given that a large number of sequence variants might be identified by NGS that are not disease causing, rigorous analytic and clinical validation is required before widespread clinical application is begun (Prior, 2014).
Similar attention is paid to assessing the male partner’s potential contribution to infertility (American Society for Reproductive Medicine, 2012b). Questions include abnormalities in pubertal development and sexual function. Erectile dysfunction, particularly in conjunction with decreased beard growth, may suggest decreased testosterone levels. Ejaculatory problems are also evaluated, including a search for developmental anomalies such as hypospadias, which could result in suboptimal semen deposition (Benson, 1997).
Sexually transmitted diseases or frequent genitourinary infections, including epididymitis or prostatitis, may lead to vas deferens inflammation and obstruction. Similarly, mumps in an adult can create testicular inflammation and damage spermatogenic stem cells (Beard, 1977). Prior cryptorchidism, testicular torsion, or testicular trauma may suggest abnormal spermatogenesis (Anderson, 1990; Cobellis, 2014). Compared with fertile males, males with unilateral or bilateral cryptorchidism have fertility rates of 80 percent and 50 percent, respectively (Lee, 1993). The reason for poor semen characteristics in these patients is unclear. The relatively warm intraabdominal temperature may cause permanent stem cell damage. Alternatively, genetic abnormalities that led to the abnormal testis location may also affect sperm production.
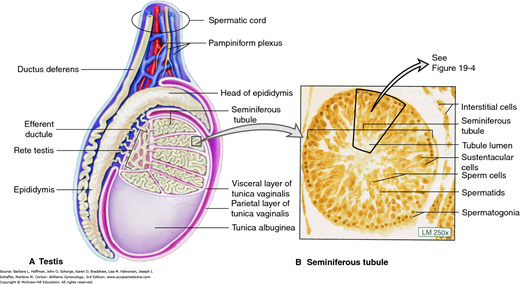
A history of varicocele is also obtained. A varicocele consists of dilated veins of the pampiniform plexus of the spermatic cords that drain the testes (Figs. 19-2 and 19-3). Varicoceles are believed to raise scrotal temperature, however, the negative affects of varicoceles on fertility are controversial (American Society for Reproductive Medicine, 2014b; Baazeem, 2011; Jarow, 2001). Although 30 to 40 percent of men seen in infertility clinics are diagnosed with a varicocele, nearly 20 percent of men in the general population are similarly affected. If a varicocele is suspected, it should be evaluated by a urologist, preferably one with a specific interest in infertility.
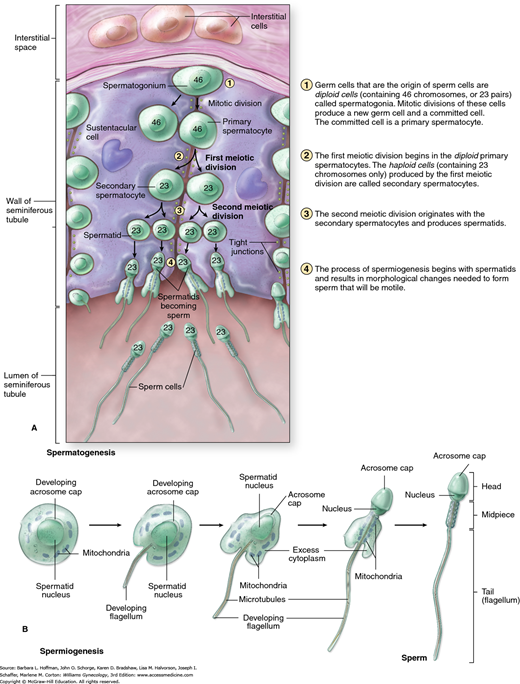
Spermatogenesis, from stem cell to mature sperm, takes nearly 90 days (Fig. 19-4). Thus, any detrimental event in the prior 3 months can adversely affect semen characteristics (Hinrichsen, 1980; Rowley, 1970). Spermatogenesis is optimal at temperatures slightly below body temperature, hence the location of the testes outside of the pelvis. Illness with high fevers or chronic hot tub use can temporarily impair sperm quality. There is no definitive evidence that boxer underwear is advantageous.
FIGURE 19-4
Male testis. A. Cutaway of the seminiferous tubule shows the mitotic and meiotic divisions involved with spermatogenesis. B. Structural changes required during spermiogenesis, as sperm cells become spermatids. (Reproduced with permission from McKinley M, O’Loughlin VD: Human Anatomy. New York: McGraw-Hill; 2006.)
Medical questions focus on prior chemotherapy or local radiation treatment that may damage spermatogonial stem cells. Hypertension, diabetes mellitus, and neurologic disorders can be associated with erectile dysfunction or retrograde ejaculation. Several medications are known to worsen semen characteristics, including cimetidine, erythromycin, gentamicin, tetracycline, and spironolactone (Sigman, 1997). Moreover, obesity, cigarettes, alcohol, illicit drugs, and environmental toxins all adversely affect semen parameters (Muthusami, 2005; Ramlau-Hansen, 2007). The increasing use of anabolic steroids also decreases sperm production by suppressing the output of intratesticular testosterone (Gazvani, 1997). Although the effects of many medications are reversible, anabolic steroid abuse may lead to lasting or even permanent damage to testicular function.
PHYSICAL EXAMINATION
A physical examination may provide many clues to the cause of infertility. Vital signs, height, and weight are recorded. A particularly short stature may reflect a genetic condition such as Turner syndrome. Hirsutism, alopecia, or acne indicates the need to measure androgen levels. Acanthosis nigricans is consistent with insulin resistance associated with PCOS or much less commonly, Cushing syndrome. Galactorrhea is often indicative of hyperprolactinemia. Additionally, thyroid abnormalities are sought. Many of these diagnoses and their management are discussed in greater detail in other chapters (Table 19-4).
| Etiology | Diagnosis | Chapter Title | Chapter Number |
| Ovulatory dysfunction | PCOS | PCOS and Hyperandrogenism | Chapter 17 |
| Hypothalamic-pituitary | Amenorrhea | Chapter 16 | |
| Age-related | Menopausal Transition | Chapter 21 | |
| POF | Amenorrhea | Chapter 16 | |
| Tubal disease | PID | Gynecologic Infection | Chapter 3 |
| Uterine abnormalities | Congenital | Anatomic Disorders | Chapter 18 |
| Leiomyomas | Pelvic Mass | Chapter 9 | |
| Asherman syndrome | Amenorrhea | Chapter 16 | |
| Other | Endometriosis | Endometriosis | Chapter 10 |
A pelvic examination may be particularly informative. Inability to place a speculum through the introitus may raise doubts about coital frequency. The vagina should be moist and rugated, and the cervix should have a reasonable amount of mucus. Both indicate adequate estrogen production. An enlarged or irregularly shaped uterus may reflect leiomyomas, whereas a fixed uterus suggests pelvic scarring due to endometriosis or prior pelvic infection. Uterosacral nodularity or ovarian masses may additionally implicate endometriosis or less commonly, malignancy.
All women should have cervical cancer screening that is up-to-date prior to treatment. Negative cultures for Neisseria gonorrhoeae and Chlamydia trachomatis are obtained to ensure that cervical manipulation during evaluation and treatment does not cause ascending infection. The breast examination must be normal, and when indicated by age or family history, a mammogram is obtained prior to initiating hormonal treatment.
Most gynecologists will not feel comfortable performing a complete male physical examination. Nevertheless, parts of this evaluation are relatively easy to perform, and a gynecologist at minimum should understand the primary focus of the examination. As signs of testosterone production, normal secondary sexual characteristics such as beard growth, axillary and pubic hair, and perhaps male pattern balding should be present. Gynecomastia or eunuchoid habitus may suggest Klinefelter syndrome (47,XXY karyotype) (De Braekeleer, 1991).
The penile urethra should be at the glans tip for proper semen deposition in the vagina. Testicular length measures at least 4 cm and a minimal testicular volume is 20 mL (Charny, 1960; Hadziselimovic, 2006). Small testes are unlikely to produce normal sperm numbers. A testicular mass may indicate testicular cancer, which can present as infertility. The epididymis should be soft and nontender to exclude chronic infection. Epididymal fullness may suggest vas deferens obstruction. The prostate should be smooth, nontender, and normal size. Additionally, the pampiniform plexus of veins is palpated for varicocele (Jarow, 2001). Importantly, both vasa deferentia should be palpable. Congenital bilateral absence of the vas deferens is associated with mutation in the gene responsible for cystic fibrosis and is discussed on page 444 (Anguiano, 1992).
EVALUATION FOR ANOVULATION
The infertility evaluation can be conceptually simplified into confirmation of: (1) ovulation, (2) normal female reproductive tract anatomy, and (3) normal semen characteristics. The specifics regarding evaluation of each of these categories are detailed in the following sections and shown in Table 19-5.
| Etiology | Evaluation |
| Ovulatory dysfunction | Ovulation predictor kit |
| Early follicular FSH ± estradiol level (ovarian reserve)± Antimüllerian hormone | |
| ± Serum measurements (TSH, prolactin, androgens) | |
| ± Ovarian sonography (antral follicle count) | |
| Tubal/pelvic disease | Hysterosalpingography |
| Laparoscopy + chromotubation | |
| Uterine factors | Hysterosalpingography |
| Transvaginal sonography/saline-infusion sonography | |
| ± Magnetic resonance imaging | |
| Hysteroscopy ± laparoscopy | |
| Male factor | Semen analysis |
Of these, ovulation may be perturbed by abnormalities within the hypothalamus, anterior pituitary, or ovaries. Hypothalamic disorders may be acquired or inherited. Acquired disorders include those due to lifestyle, for example, excessive exercise, eating disorders, or stress. Alternatively, dysfunction or improper migration of the hypothalamic gonadotropin-releasing hormone neurons may be inherited, such as that which occurs in idiopathic hypogonadotropic hypogonadism (IHH) or Kallman syndrome. Thyroid disease and hyperprolactinemia may also contribute to menstrual disturbances. A full discussion of endocrine-related disorders that result in menstrual disturbances is found in Chapter 16.
A patient’s menstrual history is an excellent predictor of regular ovulation. A woman with cyclic menses at an interval of 25 to 35 days and duration of bleeding of 3 to 7 days is most likely ovulating. Although these numbers vary widely, each woman will have her own normal pattern. Therefore, these figures typically do not vary significantly across cycles for an individual woman.
Probable ovulation is also suggested by mittelschmerz, which is midcycle pelvic pain associated with ovulation, or by moliminal symptoms such as breast tenderness, acne, food cravings, and mood changes. Ovulatory cycles are more likely to be associated with dysmenorrhea. Severe dysmenorrhea may suggest endometriosis.
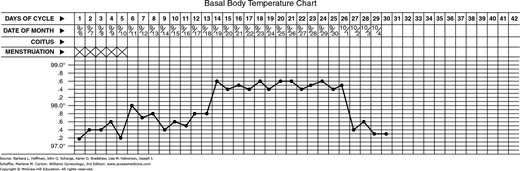
Basal body temperature (BBT) charting has long been used to identify ovulation. This test requires that a woman’s morning oral temperature be graphically charted (Fig. 19-5). Oral temperatures are usually 97.0° to 98.0°F during the follicular phase. A postovulatory rise in progesterone levels increases basal temperature by approximately 0.4° to 0.8°F. This biphasic temperature pattern is strongly predictive of ovulation (Bates, 1990). Nevertheless, although this test has the advantage of being inexpensive, it is insensitive in many women. Furthermore, for a couple wishing to conceive, the temperature increase follows ovulation, and therefore the window of maximal fertility has been missed (Luciano, 1990). Although this method is discussed here for completeness, most patients are better served by the use of the sensitive and readily available urinary ovulation detection kits described in the next section.
These kits measure urinary luteinizing hormone (LH) concentration by colorimetric assay. They are widely available in pharmacies, are relatively easy to use, and provide clear instructions regarding interpretation. In general, a woman begins testing 2 to 3 days prior to the predicted LH surge, and testing is continued daily. There is no clear consensus regarding the optimal time of day to test. Some specialists suggest that the concentrated first morning void is a logical time. Others are concerned that this sample may provide a false-positive result and recommend testing the second morning urine. Other clinicians reason that the serum LH peak occurs in the morning and that the greatest likelihood of detecting a urinary peak would be in the late afternoon or evening. Timing is probably not critical as long as the test is performed daily, as the LH surge spans only 48 to 50 hours. In most instances, ovulation will occur the day following the urinary LH peak (Luciano, 1990; Miller, 1996).
If equivocal results are obtained, the test can be repeated in 12 hours. In one study, urine LH surge assays were estimated to have 100-percent sensitivity and 96-percent accuracy. This is undoubtedly an overestimate of typical-use results (Grinsted, 1989; Guermandi, 2001).
Adequate progesterone levels are required for endometrial preparation prior to implantation. This has led to the concept of luteal phase defect (LPD), defined as inadequate endometrial development due to suboptimal progesterone production (American Society for Reproductive Medicine, 2012f).
Midluteal phase serum progesterone levels have long been used to document ovulation, although the sensitivity of this test has been questioned. In a classic 28-day cycle, serum is obtained on cycle day number 21 following the first day of menstrual bleeding, or 7 days following ovulation. Levels during the follicular phase are generally <2 ng/mL. Values above 4 to 6 ng/mL correlate with ovulation and progesterone production by the corpus luteum (Guermandi, 2001). Progesterone is secreted as pulses, and therefore a single measurement does not indicate overall production during the luteal phase. As a result, an absolute threshold for acceptable progesterone levels has not been clearly established. Although some clinicians empirically treat any woman with a progesterone level below approximately 10 ng/mL, the utility of this approach is unproven, and it is costly. Accordingly, the midluteal progesterone level is best regarded as an acceptable test for ovulation but not an absolute indicator of adequate luteal function.
Luteal phase endometrial biopsy was hoped to reflect both corpus luteum function and endometrial response, and thereby provide more clinically relevant information than a serum progesterone level alone (Noyes, 1975). Unfortunately, the utility of this test is severely hampered by high intraobserver and interobserver variability during histologic evaluation. An out-of-phase biopsy is found nearly as frequently in fertile as in infertile women, and the overlap in incidence between the two groups is large (Balasch, 1992; Scott, 1993). In its current form, the endometrial biopsy has little predictive value and is no longer considered a routine part of infertility evaluation.
Interestingly, the timing of protein expression in the endometrial glands and stroma is being defined. Potential markers for uterine receptivity include osteopontin, cytokines (leukemia inhibitory factor, colony-stimulating factor-1, and interleukin-1), cell adhesion molecules (the integrins), ion channels, and the L-selectin ligand (Carson, 2002; Garrido-Gomez, 2014; Kao, 2003; Lessey, 1998; Petracco, 2012; Ruan, 2014). In the future, endometrial biopsies may again become part of the diagnostic evaluation if expression patterns of these proteins prove to be predictive of endometrial receptivity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree