INTRODUCTION
The word abortion derives from the Latin aboriri–to miscarry. Abortion is defined as the spontaneous or induced termination of pregnancy before fetal viability. It is thus appropriate that miscarriage and abortion are terms used interchangeably in a medical context. But, because popular use of abortion by laypersons implies a deliberate intact pregnancy termination, many prefer miscarriage for spontaneous fetal loss. Both terms will be used throughout this chapter.
TERMINOLOGY
Defining viability has significant medical, legal, and social implications as this definition provides the line that separates abortion from preterm birth. It is usually defined by pregnancy duration and fetal birthweight for statistical and legal purposes. The National Center for Health Statistics, the Centers for Disease Control and Prevention (CDC), and the World Health Organization (WHO) all define abortion as any pregnancy termination—spontaneous or induced—prior to 20 weeks’ gestation or with a fetus born weighing <500 g. Confusion may be introduced by state law criteria that define abortion more widely.
Technologic developments have revolutionized current abortion terminology. Transvaginal sonography (TVS) and precise measurement of serum human chorionic gonadotropin (hCG) concentrations help to identify extremely early pregnancies and to clarify intrauterine versus ectopic location. Ubiquitous application of these practices makes it possible to distinguish between a chemical and a clinical pregnancy. Another term, pregnancy of unknown location—PUL, aids the goal of early identification and management of ectopic pregnancy (Barnhart, 2011). Management options for ectopic gestation are described in Chapter 7. Of intrauterine pregnancies, those that end in a spontaneous abortion during the first trimester, that is, within the first 126/7 weeks of gestation, are also termed early pregnancy loss or early pregnancy failure.
Approximately half of first-trimester miscarriages are anembryonic, that is, with no identifiable embryonic elements. The previous term blighted ovum for these pregnancies has fallen out of favor. The remaining pregnancies are embryonic miscarriages, which may be further grouped as either those with chromosomal anomalies (aneuploid abortions) or those with a normal chromosomal complement (euploid abortions).
Common terms used to describe pregnancy losses are listed here and will be discussed in this chapter. They include:
Spontaneous abortion—this category includes threatened, inevitable, incomplete, complete, and missed abortion. Septic abortion is used to further classify any of these that are complicated by infection.
Recurrent abortion—this term is variably defined, but it is meant to identify women with repetitive spontaneous abortions.
Induced abortion—this term is used to describe surgical or medical termination of a live fetus that has not reached viability.
SPONTANEOUS ABORTION
More than 80 percent of spontaneous abortions occur during the first 12 weeks of gestation (American College of Obstetricians and Gynecologists, 2015). With first trimester losses, death of the embryo or fetus nearly always precedes spontaneous expulsion. Death of the conceptus is usually accompanied by hemorrhage into the decidua basalis. This is followed by adjacent tissue necrosis that stimulates uterine contractions and expulsion. An intact gestational sac is usually filled with fluid and may or may not contain an embryo or fetus.
The reported incidence of spontaneous abortion varies with the sensitivity of methods used to identify them. Wilcox and colleagues (1988) studied 221 healthy women through 707 menstrual cycles. They used highly specific assays sensitive to minute concentrations of maternal serum beta human chorionic gonadotropin (β-hCG) and found that 31 percent of pregnancies were lost after implantation. Importantly, two thirds of these early losses were clinically silent.
There are factors known to influence clinically apparent spontaneous abortion. However, it is unknown if these same factors affect clinically silent miscarriages. For example, the clinical miscarriage rate nearly doubles with maternal or paternal age greater than 40 (Gracia, 2005; Kleinhaus, 2006). Although it may seem intuitive that this difference would be similar for clinically silent miscarriages, this has not been studied.
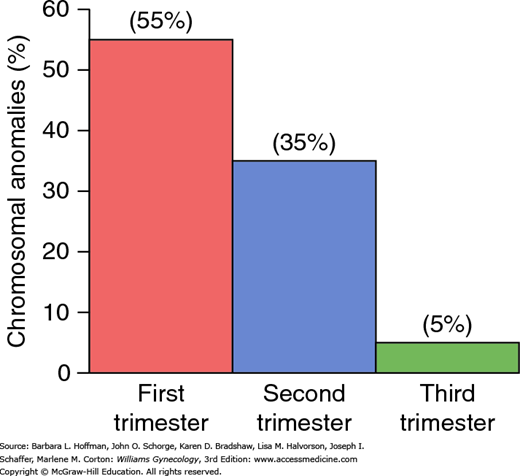
As shown in Figure 6-1, approximately half of embryonic first-trimester miscarriages are aneuploid, an incidence that decreases markedly with advancing gestation at the time of pregnancy loss. In general, aneuploid fetuses abort earlier than those with a normal chromosomal complement. Kajii (1980) reported that 75 percent of aneuploid fetuses aborted before 8 weeks, while the rate of euploid abortions peaks at approximately 13 weeks. Almost 95 percent of chromosomal abnormalities in aneuploid fetuses are caused by maternal gametogenesis errors. Thus, only 5 percent are due to aberrant paternal chromosomes (Jacobs, 1980).
FIGURE 6-1
Frequency of chromosomal anomalies in abortuses and stillbirths during each trimester. Approximate percentages for each group are shown. (Data from Eiben, 1990; Fantel, 1980; Warburton, 1980.)
Trisomy describes the condition in which three copies of a given chromosome are present. As shown in Table 6-1, autosomal trisomy is the most frequently identified chromosomal anomaly in early miscarriages. Although most trisomies result from isolated nondisjunction, balanced structural chromosomal rearrangements are present in one partner in approximately 2 percent of couples with recurrent miscarriage (Barber, 2010). Trisomies of all chromosomes have been identified with the exception of chromosome number 1. Trisomies of number 13, 16, 18, 21, and 22 are most common. Based on a study of almost 47,000 women, the baseline risk for fetal aneuploidy was 1.4 percent. One prior miscarriage increased the risk of subsequent fetal aneuploidy to 1.67 percent. Two or three previous miscarriages increased this risk to 1.8 and 2.2 percent, respectively (Bianco, 2006).
Monosomy X (45,X) is the single most common specific chromosomal abnormality and is also known as Turner syndrome. Cystic hygroma, a multiloculated lymphatic malformation, is a frequent sonographic finding with this syndrome and portends a poor prognosis. Most fetuses with monosomy X spontaneously abort, but some are liveborn females (Chap. 18). Conversely, autosomal monosomy is rare and incompatible with life.
Ploidy describes the number of complete chromosome sets. Triploidy is often associated with hydropic or molar placental degeneration (Chap. 37). Of hydatidiform moles, partial moles are characteristically triploid. Associated triploid fetuses frequently abort early, and those born later are all grossly malformed. Advanced maternal and paternal ages do not increase the incidence of triploidy. Tetraploid fetuses most often abort early in gestation and are rarely liveborn.
Chromosomal structural abnormalities infrequently cause abortion. Infants with a balanced translocation who are liveborn usually appear normal but may experience recurrent pregnancy loss as discussed on page 145.
The causes of euploid abortions are poorly understood, but various maternal medical disorders, genetic abnormalities, uterine defects, and environmental and lifestyle conditions have been implicated. Some of these, such as uterine anomalies or endocrinopathies, would be predicted to cause repetitive losses unless identified and treated. Others, such as genetic abnormalities, are not correctable. Paternal contribution to miscarriage is unclear and is discussed on page 145. Proposed etiologies will be discussed in the following sections, with a somewhat arbitrary categorization under either the isolated or recurrent pregnancy loss sections.
Pregnancy loss is clearly associated with diabetes mellitus and thyroid disorders. Beyond these, few acute or chronic diseases convey early pregnancy risk. Even developing countries report that miscarriages are rarely caused by tuberculosis, malignancies, or other serious conditions.
Anorexia nervosa and bulimia nervosa are eating disorders reported to cause subfertility, preterm delivery, and fetal growth restriction. However, their association with miscarriages is less studied (Andersen, 2009; Sollid, 2004). Chronic hypertension is a common condition associated with increased rates of preeclampsia and fetal growth restriction but may not be associated with early pregnancy loss. Inflammatory bowel disease and systemic lupus erythematosus may independently increase the risk (Al Arfaj, 2010; Khashan 2012).
Women who have had multiple miscarriages are significantly more likely to have myocardial infarctions later in life. This perhaps suggests a link with underlying vascular disease (Kharazmi, 2011). Unrepaired cyanotic heart disease is likely an abortion risk, and in some, this may persist after repair (Canobbio, 1996).
Several relatively common genital tract abnormalities—especially those of the uterus—can either prevent pregnancy implantation or disrupt a pregnancy that has implanted. Of these, congenital anomalies are most often implicated, but some acquired anomalies can also cause pregnancy loss. Unless corrected, these defects typically result in repetitive pregnancy losses and thus are considered on page 147.
As an overview, only a few organisms are proven to cause abortion. In general, systemic infections likely infect the fetoplacental unit by a blood-borne route. Others may infect locally via maternal genitourinary infection or colonization.
Chlamydia trachomatis is suspected and in one study was found in 4 percent of abortuses compared with <1 percent of controls (Baud, 2011). Oakeshott and coworkers (2002) noted an association between bacterial vaginosis and second- but not first-trimester miscarriage. One metaanalysis showed that Mycoplasma genitalium infection was significantly associated with spontaneous abortion, preterm birth, and infertility (Lis, 2015).
Data concerning the abortifacient effects of some other infections are conflicting. Namely, roles for Mycoplasma hominis, Ureaplasma urealyticum, and human immunodeficiency virus (HIV)-1 infection in abortion are unclear (Quinn 1983a,b; Temmerman, 1992; van Benthem, 2000). Moreover in livestock, several infections cause abortion, but data remain inconclusive in humans. These include Brucella abortus, Campylobacter fetus, and Toxoplasma gondii (Feldman, 2010; Hide, 2009).
Last, infections caused by Listeria monocytogenes, parvovirus, cytomegalovirus, or herpes simplex virus likely have no abortifacient effects (Brown, 1997; Feldman, 2010; Yan, 2015).
The risk of miscarriage due to a surgical procedure during pregnancy is not well studied. No currently used anesthetic agents have proven teratogenic effects when used at any gestational age. Uncomplicated surgical procedures—including abdominal or pelvic surgery—do not appear to increase the risk for abortion (Mazze, 1989). The American College of Obstetricians and Gynecologists (2013c) recommends that elective surgery be postponed until delivery or after. Nonurgent surgery should be performed in the second trimester, when possible, to decrease the theoretical risk for abortion or preterm contractions. Laparoscopy is also suitable, and adaptations for pregnancy are described in Chapter 41 (Pearl, 2011).
Ovarian tumors or cysts can be safely resected without causing pregnancy loss. An important exception involves early removal of the corpus luteum or the ovary in which it resides. If performed prior to 10 weeks’ gestation, supplemental progesterone is given. Between 8 and 10 weeks, a single injection of intramuscular 17-hydroxyprogesterone caproate, 150 mg, is given at the time of surgery. If the corpus luteum is excised between 6 to 8 weeks, then two additional 150-mg injections are given 1 and 2 weeks after the first. Other suitable progesterone replacement regimens include: (1) micronized progesterone (Prometrium) 200 or 300 mg orally once daily, or (2) 8-percent progesterone vaginal gel (Crinone), one premeasured applicator vaginally daily plus micronized progesterone, 100 or 200 mg orally once daily. Supplementation is continued until 10 weeks’ gestation.
Trauma seldom causes first-trimester miscarriage, and although Parkland Hospital is a busy trauma center, this is an infrequent association. Major trauma—especially abdominal—can cause fetal loss but is more likely as pregnancy advances.
In utero exposure to radiation may be abortifacient, teratogenic, or carcinogenic depending on the level of exposure and stage of fetal development. Threshold doses that cause abortion are not precisely known but definitely lie within the therapeutic doses used for maternal disease treatment (Williams, 2010). According to Brent (2009), exposure to <5 rads does not increase the miscarriage risk.
Female cancer survivors who were treated in the past with abdominopelvic radiotherapy may be at increased risk for miscarriage. Wo and Viswanathan (2009) reported an associated two- to eight-fold increased risk for miscarriages, low-birthweight and growth-restricted infants, preterm delivery, and perinatal mortality in women with prior radiotherapy. Hudson (2010) found an associated increased risk for miscarriage in those given radiotherapy and chemotherapy in the past for a childhood cancer.
Regarding chemotherapeutic agents, cases in which women with an early normal gestation are erroneously treated with methotrexate for an ectopic pregnancy are particularly worrisome. In a report of eight such cases, two viable-size fetuses had multiple malformations. An additional three patients spontaneously aborted their pregnancy (Nurmohamed, 2011). In a study of methotrexate treatment for rheumatic disease, the observed incidence of spontaneous abortion and major birth defects was statistically elevated in the patients receiving methotrexate after conception compared with disease-matched controls or women without autoimmune disease (Weber-Schoendorfer, 2014).
Only a few medications have been evaluated regarding the risk for early pregnancy loss. Conclusions have been difficult to derive from these studies based on multiple confounding factors including differences in doses, exposure duration, gestational age, and underlying maternal disease. Nonsteroidal antiinflammatory drugs are not linked to early pregnancy loss (Edwards, 2012). Also, oral contraceptives or spermicidal agents used in contraceptive creams and jellies are not associated with an increased miscarriage rate. When intrauterine devices fail to prevent pregnancy, however, the risk of abortion, and specifically septic abortion, increases substantively (Ganer, 2009; Moschos, 2011).
Most routine immunizations can be given safely during pregnancy. Fortunately, evidence to link immunization, even live-virus vaccines, with miscarriage is lacking. Two large metaanalyses clearly demonstrated no harm from the human papillomavirus (HPV) or influenza vaccine in early pregnancy (McMillan, 2015; Wacholder, 2010).
Dietary deficiency of any one nutrient or moderate deficiency of all nutrients does not appear to be an important cause of abortion. Even in extreme cases—for example, hyperemesis gravidarum—abortion is rare. Dietary quality may be important as this risk may be reduced in women who consume fresh fruit and vegetables daily (Maconochie, 2007).
Data also suggest that extremes in weight can be deleterious. Obesity is associated with subfertility, increases the risk of miscarriage, and results in a host of other adverse pregnancy outcomes (Boots, 2014). Bellver and associates (2010a) studied 6500 women with in vitro fertilization (IVF)-conceived pregnancies and found that pregnancy and live birth rates were reduced progressively for each body mass index (BMI) unit increase. Although the risks for many adverse late-pregnancy outcomes are decreased after bariatric surgery, any salutary effects on the miscarriage rate are not clear (Guelinckx, 2009). Pregnant women who have undergone bariatric surgery are monitored for nutritional deficiencies (American College of Obstetricians and Gynecologists, 2013d).
Low BMI has also been associated with increased miscarriage risk (Helgstrand, 2005; Metwally, 2010). A cohort of more than 90,000 women demonstrated that the primary modifiable prepregnant risk factors for miscarriage are being underweight, obese, or aged 30 years or older at conception (Feodor Nilsson, 2014).
Of these, alcohol has been well studied in pregnancy. Earlier observations were that both miscarriage and fetal anomaly rates increased with alcohol abuse rates during the first 8 weeks of gestation (Armstrong, 1992; Floyd, 1999). Such outcomes likely are dose related, although safe levels have not been identified. Maconochie (2007) observed a significantly increased risk only with regular or heavy alcohol use. Low-level alcohol consumption did not significantly increase the abortion risk in two studies (Cavallo, 1995; Kesmodel, 2002). In contrast, Danish National Birth Cohort data suggest an adjusted hazard ratio for first-trimester fetal death of 1.66 with as few as two drinks per week (Andersen, 2012).
At least 15 percent of pregnant women admit to cigarette smoking. It seems intuitive that cigarettes could cause early pregnancy loss by several mechanisms that cause adverse late-pregnancy outcomes (Catov, 2008). Some studies link smoking with abortion risk and find a dose-response effect (Armstrong, 1992; Nielsen, 2006). Conversely, several others do not support this association (Rasch, 2003; Wisborg, 2003).
Excessive caffeine consumption has been associated with an increased abortion risk. Heavy intake, or approximately five cups of coffee per day—about 500 mg of caffeine—slightly increases the abortion risk (Cnattingius, 2000). Studies of “moderate” intake—less than 200 mg daily—did not demonstrate increased risk (Savitz, 2008; Weng, 2008). Currently, the American College of Obstetricians and Gynecologists (2013b) concludes that moderate consumption likely is not a major abortion risk and that any associated risk with higher intake is unsettled.
The adverse effects of illicit drugs on early pregnancy loss also are unclear. Although cocaine was linked to increased miscarriage in one study, reanalysis refuted this conclusion (Mills, 1999; Ness, 1999).
Some environmental toxins such as benzene are implicated in fetal malformations, but data regarding miscarriage risk are less clear (Lupo, 2011). Earlier reports implicated arsenic, lead, formaldehyde, benzene, and ethylene oxide (Barlow, 1982). More recently, evidence suggests that DDT—dichlorodiphenyltrichloroethane—may raise miscarriage rates (Eskenazi, 2009). Nevertheless, DDT-containing insecticides are endorsed by the WHO (2011) for mosquito control to prevent malaria.
Few studies assess occupational exposure and abortion risks. Exposure to neither the electromagnetic fields of video display terminals nor to ultrasound increases miscarriage rates (Schnorr, 1991; Taskinen, 1990). An elevated risk has been described for dental assistants exposed to 3 or more hours of nitrous oxide per day in offices without gas-scavenging equipment (Rowland, 1995). In their metaanalysis, Dranitsaris and colleagues (2005) found a small incremental risk for spontaneous abortion in women who worked with cytotoxic antineoplastic chemotherapeutic agents.
As a group, abortion can be divided clinically several ways. Commonly used categories include threatened, inevitable, incomplete, complete, and missed abortion. When the products of conception, uterus, and other pelvic organs become infected, the term septic abortion is descriptive.
Of these, threatened abortion is presumed when there is a bloody vaginal discharge or bleeding through a closed cervical os (Hasan, 2009). In early pregnancy, bleeding is common and includes that with blastocyst implantation at the time of expected menses. Of pregnant women, approximately one quarter experience first-trimester spotting or heavier bleeding. Of these, 43 percent will subsequently miscarry. Bleeding is by far the most predictive risk factor for pregnancy loss, but this risk is substantially less if fetal cardiac activity is seen sonographically (Tongsong, 1995).
With miscarriage, bleeding usually begins first, and cramping abdominal pain follows hours to days later. There may be low-midline rhythmic cramps; persistent low backache with pelvic pressure; or dull and midline suprapubic discomfort. The combination of bleeding and pain predicts a poor prognosis for pregnancy continuation. Even if miscarriage does not follow early bleeding, the risks for later adverse pregnancy outcomes are elevated (Table 6-2). In a study of almost 1.8 million pregnancies, the risk for many of these pregnancy complications rose threefold (Lykke, 2010).
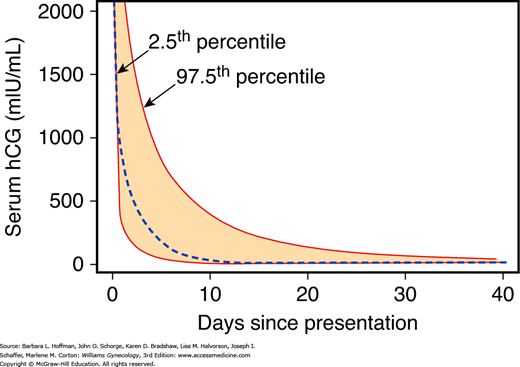
A woman with an early pregnancy, vaginal bleeding, and pain should be examined. The primary goal is prompt diagnosis of an ectopic pregnancy. Serial quantitative serum β-hCG levels, progesterone levels, and transvaginal sonography, alone or in combination, can help ascertain if the fetus is alive and if it is within the uterus. Repeat evaluations are often necessary as none of these tests has 100-percent accuracy for the diagnosis of pregnancy location or fetal viability. Figure 6-2 depicts composite serum β-hCG level disappearance curves in women with bleeding who went on to have an early miscarriage (Barnhart, 2004). Several predictive models based on serum β-hCG levels done 48 hours apart have been described (Barnhart, 2010; Condous, 2007). Of these, serum β-hCG levels with a robust uterine pregnancy should increase at least 53 to 66 percent every 48 hours (Barnhart, 2004; Kadar, 1982). Seeber and associates (2006) used an even more conservative 35-percent rise after 48 hours.
FIGURE 6-2
Composite curve describing decline in serial human chorionic gonadotropin (hCG) values starting at a level of 2000 mIU/mL following early spontaneous miscarriage. The dashed line is the predicted curve based on the summary of data from all women. The colored area within the dashed lines represent the 95-percent confidence intervals. (Data from Barnhart K, Sammel MD, Chung K, et al: Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol 104:975, 2004.)
With serum progesterone levels, those <5 ng/mL suggest a dying pregnancy. In contrast, values >20 ng/mL support the diagnosis of a healthy pregnancy. However, progesterone levels often lie between these thresholds, are then considered indeterminate, and thus are less informative.
Transvaginal sonography can document the location and viability of a gestation. If this cannot be done, then pregnancy of unknown location (PUL) is diagnosed. Notably, a consensus conference in 2012 concluded that prior sonographic criteria for fetal viability yielded unacceptably high rates of viable intrauterine pregnancies (IUPs) being falsely diagnosed as nonviable or as PULs (American College of Obstetricians and Gynecologists, 2015; Doubilet, 2014). Such erroneous diagnoses can lead to unnecessary surgical or medical treatment, interruption of a viable IUP, or incorrect assumption that a woman is at recurrent risk for an ectopic pregnancy. They proposed more stringent guidelines for the diagnosis of pregnancy failure (Table 6-3).
| Diagnostic Sonographic Findings |
| CRL ≥7 mm and no heartbeat MSD ≥25 mm and no embryo Absence of embryo with heartbeat ≥2 weeks after a scan showed a gestational sac without a yolk sac Absence of embryo with heartbeat ≥11 days after a scan showed a gestational sac with a yolk sac |
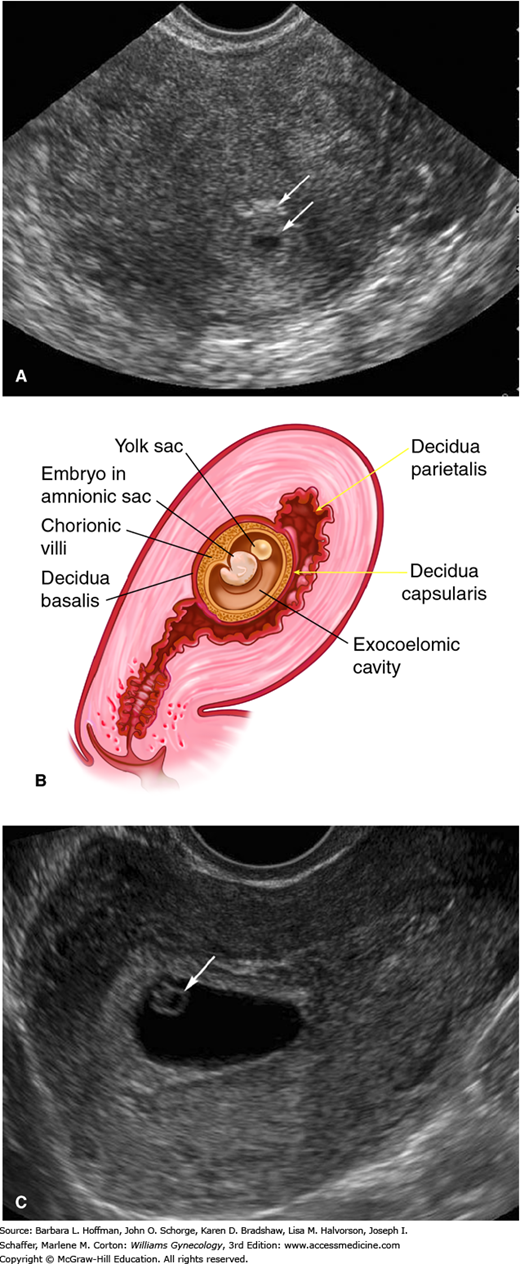
One early TVS sign of an IUP is the gestational sac. This anechoic fluid collection represents the exocoelomic cavity. It may be encircled by two echogenic external layers, the double-decidual sign, which represent the decidua parietalis and decidua capsularis (Fig. 6-3). The gestational sac can be seen by 4.5 weeks with maternal β-hCG levels between 1500 and 2000 mIU/mL (Barnhart, 1994; Timor-Tritsch, 1988). More recently, Connolly and colleagues (2013) reported that a threshold value of 3500 mIU/mL may be required to detect a gestational sac in 99 percent of cases. Importantly, a gestational sac may appear similar to other intrauterine fluid accumulations such as the pseudogestational sac (pseudosac) present with ectopic pregnancy (Fig. 7-4). A pseudosac may be excluded once a definite yolk sac or embryo is seen inside the sac. The diagnosis of an IUP should be avoided if the yolk sac is not yet seen.
FIGURE 6-3
Early intrauterine pregnancy. A. Sonogram shows the anechoic gestational sac surrounded by two concentric echogenic layers, which are the inner decidua capsularis (arrow) and the peripheral decidua parietalis (arrow). B. The drawing shows the anatomy of an early pregnancy. C. The yolk sac (arrow) is circular and anechoic, and in this image, it lies to the right of its adjacent embryo.
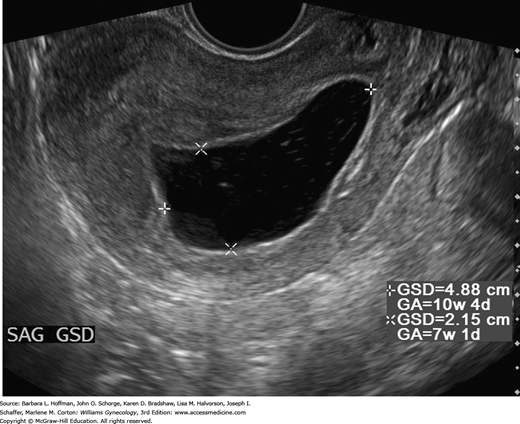
The yolk sac is a circular, 3- to 5-mm-diameter anechoic structure. It is typically seen within the gestational sac at approximately 5.5 weeks’ gestation and with a mean sac diameter (MSD) ≥10 mm. At approximately 6 weeks’ gestation, a 1- to 2-mm embryo adjacent to the yolk sac can be found (see Fig. 6-3). Absence of an embryo in a sac with a MSD of 16 to 24 mm is suspicious for pregnancy failure (Doubilet, 2014). Cardiac motion can be detected at 6 to 6.5 weeks’ gestation, at an embryonic length of 1 to 5 mm. As shown in Table 6-3, absent cardiac activity at certain stages can be used to diagnose pregnancy failure.
Amnionic fluid leaking through a dilated cervix portends almost certain abortion. Either uterine contractions begin promptly or infection develops. Rarely is a gush of vaginal fluid during the first half of pregnancy without serious consequence.
In the rare case, fluid may have collected previously between the amnion and chorion and may not be associated with pain, fever, or bleeding. If documented, then diminished activity with observation is reasonable for some early-to-mid second-trimester gestations. After 48 hours, if no additional amnionic fluid has escaped and if there is no bleeding, cramping, or fever, then a woman may resume ambulation and pelvic rest. With bleeding, cramping, or fever, abortion is considered inevitable, and the uterus is evacuated.
Bleeding that follows partial or complete placental separation and that is coupled with dilation of the cervical os is termed incomplete abortion. The fetus and the placenta may remain entirely within the uterus or partially extrude through the dilated os. Before 10 weeks, they are frequently expelled together, but later, they deliver separately. Management options of incomplete abortion include curettage, medical abortion, or expectant management in clinically stable women as discussed on page 152. With surgical therapy, additional cervical dilatation may be necessary before suction curettage. In others, retained placental tissue simply lies loosely within the cervical canal and allows easy extraction with ring forceps. With miscarriage, removed products of conception are sent to pathology for standard histologic analysis. With this, products of conception are confirmed, and gestational trophoblastic disease is excluded.
In some cases, expulsion of the entire pregnancy is completed before a patient presents for care. In such cases, a history of heavy bleeding, cramping, and tissue passage at home is common. Physical examination reveals a closed cervical os. Patients are encouraged to bring in passed tissue, which may be a complete gestation, blood clots, or a decidual cast. The last is a layer of endometrium in the shape of the uterine cavity that when sloughed can appear as a collapsed sac (Fig. 7-7).
If a gestational sac is not identified grossly in the expelled specimen, sonography is performed to differentiate a complete abortion from threatened abortion or ectopic pregnancy. Characteristic findings of a complete abortion include a thickened endometrium without a gestational sac. However, this does not guarantee a recent IUP. Condous and associates (2005) described 152 women with heavy bleeding, an empty uterus with endometrial thickness <15 mm, and a diagnosis of completed miscarriage. Six percent were subsequently proven to have an ectopic pregnancy. Thus, a diagnosis of complete abortion should not be made unless an intrauterine pregnancy was previously diagnosed sonographically or passage of a gestational sac has been confirmed. In unclear settings, serial serum β-hCG measurements aid correct diagnosis. With complete abortion, these levels drop quickly (Connolly, 2013).
The term missed abortion requires clarification. Historically, the term was used to describe dead products of conception that were retained for weeks or months in a uterus with a closed cervical os. Despite this, concurrent early pregnancy findings of amenorrhea, nausea and vomiting, breast changes, and uterine growth appeared normal. To elucidate these disparities, Streeter (1930) studied aborted fetuses and observed that the mean interval from death-to-abortion was approximately 6 weeks.
This historical description of missed abortion is in contrast to that defined currently based on results of serial serum β-hCG assays and TVS (Fig. 6-4). There is rapid confirmation of fetal or embryonic death—even in early pregnancies—and many women choose uterine evacuation when the diagnosis is confirmed. Many classify these as a missed abortion, although the term is used interchangeably with early pregnancy loss or pregnancy wastage (Silver, 2011).
Horrific infections and maternal deaths associated with criminal septic abortions have become rare with legalized abortion. Still, perhaps 1 to 2 percent of women with threatened or incomplete miscarriage can develop infection and sepsis syndrome. Elective abortion, either surgical or medical, is also occasionally complicated by severe and even fatal infections (Barrett, 2002; Ho, 2009). Bacteria gain uterine entry and colonize dead conception products. Organisms may invade myometrial tissues and extend to cause parametritis, peritonitis, septicemia, and rarely, endocarditis (Vartian, 1991).
Significant necrotizing infections and toxic shock syndrome have been reported due to Clostridium perfringens, Clostridium sordellii, and group A streptococcus—S pyogenes (Centers for Disease Control and Prevention, 2005; Daif, 2009). Clinical manifestations begin within a few days after the abortion.
Women may be afebrile when first seen with prominent endothelial injury, capillary leakage, hemoconcentration, hypotension, and a profound leukocytosis (Fischer, 2005; Ho, 2009). Maternal deaths from these clostridial species approximate 0.58 per 100,000 medical abortions (Meites, 2010).
Treatment of infected abortion or postabortal sepsis includes prompt administration of broad-spectrum antibiotics. Suitable regimens are found in Table 3-20. For women with septic incomplete abortion or for those with retained fragments, intravenous antimicrobial therapy is promptly followed by uterine evacuation. Most women respond to this treatment within 1 to 2 days and are discharged when afebrile. Continued oral antibiotic treatment is likely unnecessary (Savaris, 2011). Rarely, sepsis causes acute respiratory distress syndrome, acute kidney injury, or disseminated intravascular coagulopathy. In these cases, intensive supportive care is essential.
To prevent postabortal sepsis, prophylactic antibiotics are given at the time of surgical evacuation or induced abortion. The American College of Obstetricians and Gynecologists (2014b) recommends doxycycline, 100 mg orally 1 hour prior to and then 200 mg orally after the procedure.
Unless there is serious bleeding or infection, management of spontaneous abortion can be individualized. In the case of threatened abortion, bed rest is often recommended but does not improve outcomes. Neither does treatment with a host of medications that include chorionic gonadotropin (Devaseelan, 2010). Acetaminophen-based analgesia will help relieve discomfort from cramping.
For other cases of spontaneous abortion, any of three management options is reasonable—expectant, medical, or surgical. Each has its own risks and benefits. For example, the first two are associated with unpredictable bleeding, and some women will require unscheduled curettage. Nevertheless, expectant management for suspected first-trimester miscarriage results in spontaneous resolution of pregnancy in more than 80 percent of women (Luise, 2002). Whereas surgical treatment is definitive and predictable, it is invasive and not necessary for all women (American College of Obstetricians and Gynecologists, 2015).
With persistent or heavy bleeding, the hematocrit is determined. If there is significant anemia or hypovolemia, then pregnancy evacuation is generally indicated. In cases in which there is a live fetus, some may infrequently choose transfusion and further observation.
Several randomized studies that compared these management schemes were reviewed by Neilson (2013). A major drawback cited for between-study comparisons was varied inclusion criteria and techniques. For example, the success of medical therapy was enhanced in studies that included women with vaginal bleeding compared with those that excluded such women (Creinin, 2006). Selected studies reported since 2005 are listed in in Table 6-4. These permit some generalizations. First, success is dependent on the type of early pregnancy loss, that is, incomplete versus missed abortion. Second, expectant management of spontaneous incomplete abortion has failure rates as high as 50 percent. Medical therapy failure rates with prostaglandin E1 (PGE1) may be related to dose, route, and form, and rates vary from 5 to 40 percent. Last, curettage results in a quick resolution that is 95- to 100-percent successful. Importantly, subsequent pregnancy rates do not differ among these management methods (Smith, 2009).
| Study | Inclusion Criteria | No. | Treatment Arms | Outcomes |
| Nguyen (2005) | Incomplete SAB | 149 | (1) PGE1, 600 μg orally (2) PGE1, 600 μg orally initially and at 4 hour | 60% completed at 3 d 95% at 7 d; 3% curettage |
| Zhang (2005) | Pregnancy failure a | 652 | (1) PGE1, 800 μg vaginally (2) Vacuum aspiration | 71% completed at 3 d; 16% failure 97% successful |
| Trinder (2006) (MIST Trial) | Incomplete SAB; missed AB | 1200 | (1) Expectant (2) PGE1, 800 μg vaginally ±200 mg mifepristone (3) Suction curettage | 50% curettage 38% curettage 5% repeat curettage |
| Dao (2007) | Incomplete SAB | 447 | (1) PGE1, 600 μg orally (2) Vacuum aspiration | 95% completed 100% completed |
| Torre (2012) | First-trimester miscarriage b | 174 | (1) Immediate–PGE1, 200 μg orally Day 2—400 μg vaginally (2) Delayed—no treatment; TVS days 7 and 14 | 81% completed 19% curettage 57% completed 43% curettage |
During spontaneous miscarriage, 2 percent of D-negative women will become isoimmunized if not provided passive isoimmunization. With an induced abortion, this rate may reach 5 percent. The American College of Obstetricians and Gynecologists (2013e) recommends anti-Rh0(D)immunoglobulin given as 300 μg intramuscularly (IM) for all gestational ages. Alternatively, dosing may be graduated, with 50 μg given IM for pregnancies ≤12 weeks and 300 μg given for those ≥13 weeks.
Prophylaxis with a threatened abortion is controversial, and recommendations are limited by scarce evidence-based data (Hannafin, 2006; Weiss, 2002). Up to 12 weeks’ gestation, prophylaxis is optional for women with threatened abortion and a live fetus. At Parkland Hospital, we administer a 50-μg dose to all D-negative women with first-trimester bleeding.
RECURRENT MISCARRIAGE
Terms used to describe repetitive early spontaneous pregnancy losses include recurrent miscarriage, recurrent spontaneous abortion, and recurrent pregnancy loss, with the last term gaining popularity. The term habitual abortion was used in the past and currently is not preferred. Approximately 1 to 2 percent of fertile couples experience recurrent miscarriage, which is classically defined as three or more consecutive losses at <20 weeks’ gestation or with a fetal weight <500 g. Most women with recurrent miscarriage have embryonic or early fetal loss. Recurrent anembryonic miscarriage or those with consecutive losses after 14 weeks are much less common.
Studies are difficult to compare due to a lack of standard definitions. Some investigators include women with two rather than three consecutive losses, whereas others include women with three nonconsecutive losses. Documentation of pregnancy with β-hCG levels, sonography, and/or pathological examination also varies. At minimum, recurrent miscarriage should be distinguished from sporadic pregnancy loss, which implies intervening pregnancy that reached viability.
As shown in Table 6-5, the success rate of a subsequent viable pregnancy decreases as age increases and as the number of consecutive losses increases (Brigham, 1999). Following more than 150,000 miscarriages, Bhattacharya and coworkers (2010) reported miscarriage rates as they related to the number of prior losses (Table 6-6). In both studies, the risk for subsequent miscarriage was similar following either two or three losses.
| Previous Pregnancy Losses | ||||
| 0 | 1 | 2 | 3 | |
| Pregnancies (n) | 143,595 | 6577 | 700 | 115 |
| Subsequent risk for miscarriage | 7.0% | 13.9% | 26.1% | 27.8% |
The American Society for Reproductive Medicine (2013) has proposed that recurrent pregnancy loss (RPL) be defined by two or more failed clinical pregnancies confirmed by either sonographic or histopathologic examination. Each loss should be considered an impetus for further evaluation, and a thorough evaluation is warranted after three losses. Other considerations include maternal age and the interval between pregnancies. Evaluation and treatment are considered earlier in couples with concordant subfertility. This practice is further justified by a recent study of more than 1000 women in which those with two pregnancy losses had a prevalence of abnormal test findings similar to that of women with three or more losses (Jaslow, 2010). Remarkably, the chances for a successful pregnancy are more than 50 percent even after five losses in women younger than 45 years (Brigham, 1999).
Of the many putative causes of early RPL, only three are widely accepted: parental chromosomal abnormalities, antiphospholipid antibody syndrome, and acquired or congenital uterine abnormalities. Other suspected but not proven causes are alloimmunity, endocrinopathies, and environmental toxins. As discussed on page 139, very few infections are firmly associated with early pregnancy loss. It is even less likely that infections would cause recurrent miscarriage because most are sporadic or they stimulate protective maternal antibodies.
The timing of the recurrent losses may provide a clue to their etiology. For a given individual with RPL, each miscarriage tends to occur near the same gestational age (Heuser, 2010). Genetic factors most frequently result in early embryonic losses, whereas autoimmune or anatomic abnormalities more likely lead to second-trimester losses. Although many causes of RPL parallel those of sporadic miscarriage, the relative incidence differs between the two categories. For example, recurrent first-trimester losses have a significantly lower incidence of genetic abnormalities than observed in sporadic losses. In one series, the products of conception had a normal karyotype in half of recurrent miscarriages but in only a fourth of sporadic losses (Sullivan, 2004).
Although these account for only 2 to 5 percent of RPL, karyotype evaluation of both parents is recommended (American Society for Reproductive Medicine, 2012). Data from 8000 couples with two or more miscarriages demonstrated structural chromosomal anomalies in 3 percent—a fivefold greater incidence than observed for the general population. In the parents, balanced reciprocal translocations accounted for 50 percent of identified abnormalities; robertsonian translocations for 24 percent; and X chromosome mosaicism such as 47, XXY—Klinefelter syndrome—for 12 percent. Inversions and various other anomalies made up the remainder. The women were twice as likely as the men to harbor the cytogenetic abnormality (Tharapel, 1985
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree