INTRODUCTION
Today, an ever-increasing variety of effective methods is available for fertility regulation. Although none is completely without side effects or potential danger, it remains axiomatic that contraception poses fewer risks than pregnancy (Table 5-1). Contraceptive availability is paramount for the care of women, as approximately half of pregnancies in the United States are unintended (Finer, 2014). Moreover, half of these women are using contraception at the time of conception (Henshaw, 1998). These statistics have prompted a reexamination of contraceptive counseling to prevent unplanned pregnancy (American College of Obstetricians and Gynecologists, 2011; Steiner, 2006).
| Method | 15–24 Years | 25–34 Years | 35–44 Years |
| Pregnancy | 5.1 | 5.5 | 13.4 |
| Abortion | 2.0 | 1.8 | 13.4 |
| Intrauterine device | 0.2 | 0.2 | 0.4 |
| Rhythm, withdrawal | 1.3 | 1.0 | 1.3 |
| Barrier method | 1.0 | 1.3 | 2.0 |
| Spermicides | 1.8 | 1.7 | 2.1 |
| Oral contraceptives | 1.1 | 1.5 | 1.4 |
| Implants/injectables | 0.4 | 0.6 | 0.5 |
| Tubal sterilization | 1.2 | 1.1 | 1.2 |
| Vasectomy | 0.1 | 0.1 | 0.1 |
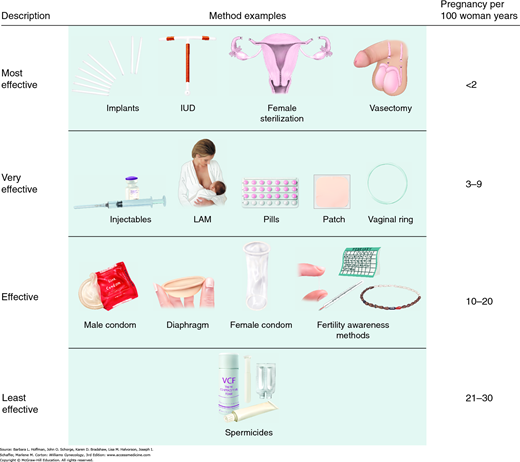
Methods are now grouped according to their effectiveness. Top-tier or first-tier methods are those that are most effective and are characterized by their ease of use (Fig. 5-1). These methods require only minimal user motivation or intervention and have an unintended pregnancy rate less than 2 per 100 women during the first year of use (Table 5-2). As expected, these first-tier methods provide the longest duration of contraception after initiation and require the fewest number of return visits. Top-tier methods include intrauterine contraceptive devices, contraceptive implants, and various methods of male and female sterilization. A reduction in unintended pregnancies can be better achieved by increasing top-tier method use. Thus, although counseling is provided for all contraceptive methods, common misperceptions regarding some of the top-tier methods—especially intrauterine contraception—can also be dispelled.
| Methoda | Perfect Use | Typical Use |
| Top Tier: Most Effective | ||
| Intrauterine devices: | ||
| Levonorgestrel system | 0.2 | 0.2 |
| T 380A copper | 0.6 | 0.8 |
| Levonorgestrel implants | 0.05 | 0.05 |
| Female sterilization | 0.5 | 0.5 |
| Male sterilization | 0.1 | 0.15 |
| Second Tier: Very Effective | ||
| Combination pill | 0.3 | 9 |
| Vaginal ring | 0.3 | 9 |
| Patch | 0.3 | 9 |
| DMPA | 0.2 | 6 |
| Progestin-only pill | 0.3 | 9 |
| Third Tier: Effective | ||
| Condom | ||
| Male | 2 | 18 |
| Female | 5 | 21 |
| Diaphragm with spermicides | 6 | 12 |
| Fertility-awareness | 24 | |
| Standard days | 5 | |
| Two day | 4 | |
| Ovulation | 3 | |
| Symptothermal | 0.4 | |
| Fourth Tier: Least Effective | ||
| Spermicides | 18 | 28 |
| Sponge | ||
| Parous women | 20 | 24 |
| Nulliparous women | 9 | 12 |
| No WHO Category | ||
| Withdrawal | 4 | 22 |
| No contraception | 85 | 85 |
Second-tier methods include systemic hormonal contraceptives that are available as oral tablets, intramuscular injections, transdermal patches, or transvaginal rings. In sum, their expected failure rate is 3 to 9 percent per 100 users during the first year. This higher rate likely reflects failure to redose at the appropriate interval. Automated reminder systems for these second-tier methods have been repeatedly shown to have limited efficacy (Halpern, 2013).
Third-tier methods include barrier methods for men and women and fertility awareness methods such as cycle beads. Their expected failure rate is 10 to 20 percent per 100 users in the first year. However, efficacy increases with consistent and correct use.
Fourth-tier methods include spermicidal preparations, which have a failure rate of 21 to 30 percent per 100 first-year users. The withdrawal method is so unpredictable that some conclude that it does not belong among other contraceptive methods (Doherty, 2009).
MEDICAL ELIGIBILITY CRITERIA
The World Health Organization (WHO) (2010) has provided evidence-based guidance for the use of all highly effective reversible contraceptive methods by women with various health factors. These guidelines were intended to be modified by individual countries to best serve their populations specific circumstances. Thus, the Centers for Disease Control and Prevention (2010, 2011) published United States Medical Eligibility Criteria (US MEC) for contraceptive use in the United States. These US MEC guidelines are available and updated regularly at the CDC website: http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/USMEC.htm. In the US MEC, many contraceptive methods are classified into six groups by their similarity: combination oral contraceptive (COC), progestin-only pill (POP), depot medroxyprogesterone acetate (DMPA), implants, levonorgestrel-releasing intrauterine system (LNG-IUS), and copper intrauterine device (Cu-IUD). For a given health condition, each method is categorized 1 through 4. The score describes a method’s safety profile for a typical woman with that condition: (1) no restriction of method use, (2) method advantages outweigh risks, (3) method risks outweigh advantages, or (4) method poses an unacceptably high health risk.
Among others, lactation is one factor addressed in the US MEC guidelines. Approximately 20 percent of breast-feeding women will ovulate by 3 months postpartum. Ovulation often precedes menstruation, and these women are at risk for unplanned pregnancy. For women who breast feed intermittently, effective contraception should begin as if they were not breast feeding. Moreover, contraception is essential after the first menses unless pregnancy is planned.
Of available methods, Cu-IUD in breast-feeding women has a category 1 or 2 rating (Table 5-3). Women are counseled that effects of the etonogestrel-releasing contraceptive implant (Nexplanon) or LNG-IUS and breast feeding are not known, but studies have mostly shown no adverse association (Gurtcheff, 2011). Because POPs have little effect on lactation, they are also preferred by some for use up to 6 months in women who are exclusively breast feeding. According to the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012), POPs and DMPA may be initiated prior to discharge regardless of breast-feeding status. For the etonogestrel implant, insertion is delayed until 4 weeks postpartum for those exclusively breast-feeding but can be inserted anytime for those not nursing. Combination hormone contraception may begin at 6 weeks following delivery, if breast feeding is well established and the infant’s nutritional status is surveilled. The CDC (2011) revised the US MEC guidelines regarding the use of combined hormonal contraception during the puerperium due to the higher risk of venous thromboembolism (VTE) during these weeks.
| Method a | Category | Comments |
| CHCs b | ||
| Breastfeeding <1 month >1 month | 3 2 | Evidence limited. Guidelines based on theoretical concerns |
| Non-breastfeeding <21 days 21–42 days, with risksc 21–42 days, with no risks >42 days | 4 3 2 1 | Theoretical concerns for thrombosis risks. Blood coagulation and fibrinolysis virtually normalized by 3 weeks pp |
| DMPA, POPs, Implants | ||
| Breastfeeding <1 month >1 month | 2 1 | Theoretical concerns that early use may diminish breast milk production are not supported by evidence. Limited studies |
| Non-breastfeeding | 1 | Limited evidence suggests no adverse side effects |
| LNG-IUS | ||
| Breastfeeding or not <10 mins 10 mins to ≤4 wks ≥4 wks Puerperal sepsis | 2 2 1 4 | Theoretical risk of diminished breast milk production. Minimal evidence IUD insertion could worsen condition |
| Cu-IUD | ||
| Breastfeeding or not <10 min 10 min to ≤4 wks ≥4 wks | 1 2 1 | IUD placement <10 min pp is associated with lower expulsion rates compared with later IUD placement up to >72 hr pp. No comparative data for insertion >72 hr pp At c-section, postplacental placement associated with lower expulsion rate than after vaginal delivery No increased risk of infection or perforation associated with pp insertion |
| Puerperal sepsis | 4 | IUD insertion could worsen condition |
Concerns regarding contraceptive steroids and use with breast feeding are based on the theoretical and biologically plausible—but unproven—possibility that systemic progestins may interfere with initial breast milk production. Importantly, contraceptive steroids are not purported to harm the quality of breast milk. Minute quantities of the hormones are excreted in breast milk, but no adverse effects on infants have been reported. In two reviews, authors describe the lack of evidence to support a negative impact of hormonal contraception on lactation (Tepper, 2015; Truitt, 2003). The reviewers concluded that all the studies were of poor to fair quality and that randomized trials are needed.
Females at both ends of the reproductive spectrum have unique contraceptive needs, which are discussed in Chapters 14 and 21. With adolescents, since the mid-1800s, the age of menarche has dropped. Thus, reproductive function is established many years earlier than psychosocial comprehension regarding the consequences of sexual activity. Such early sexual development may result in intermittent spontaneous sexual encounters with a naïve perception of pregnancy and sexually transmitted disease (STD) risks (Sulak, 1993). Importantly, adolescents have unintended pregnancy rates that approach 85 percent (Finer, 2014). Thus, effective contraception counseling ideally is provided before the onset of sexual activity. In most states, minors have explicit legal authority to consent to contraceptive services, and in many areas, publicly funded clinics provide free contraception to adolescents (Guttmacher Institute, 2014). Moreover, contraception may be provided without a pelvic examination or cervical cancer screening.
In the perimenopause, ovulation becomes irregular and fertility wanes. However, pregnancies do occur, and in women aged >40 years, nearly half of all pregnancies are unintended (Finer, 2011). Importantly, pregnancy with advanced maternal age carries an increased risk for pregnancy-related morbidity and mortality. Women in this group may also have coexistent medical problems that may preclude certain contraceptive methods. Finally, perimenopausal symptoms may be present in this group and may be improved with hormonal contraceptive methods.
TOP-TIER CONTRACEPTIVE METHODS
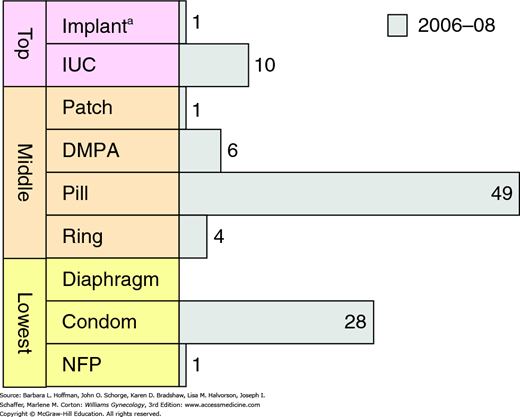
Fears and concerns with legal liability caused this method to become almost obsolete. However, intrauterine contraception (IUC) has again gained popularity, and IUC use increased from 2 percent in 2002 to 10 percent in 2008 (Fig. 5-2) (Mosher, 2010). Still, this is much lower compared with the worldwide IUC use rate of 14 percent, and specifically with that of China (40 percent) and northern Europe (11 percent) (United Nations, 2013).
Some barriers to IUC use in the United States include cost, politics, and provider failure to offer or encourage use of this method. To reduce the high proportion of unplanned pregnancies, the American College of Obstetricians and Gynecologists (2013b) encourages use of long-acting reversible contraceptives (LARC) for all appropriate candidates, including adolescents. Despite higher up-front costs, the extended span of effective IUC use results in competitive cost effectiveness compared with other contraceptive forms.
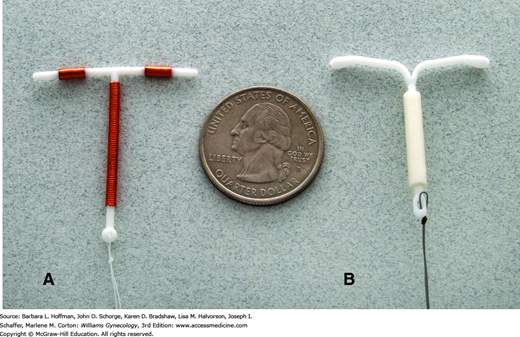
Three levonorgestrel-releasing intrauterine contraceptives are Food and Drug Administration (FDA)-approved in the United States. Named Mirena, Skyla, and Liletta, devices are T-shaped polyethylene structures with the stem encased by a cylinder containing polydimethylsiloxane and levonorgestrel (Fig. 5-3). The cylinder has a permeable membrane that regulates continuous daily hormone release. The Mirena is currently approved for 5 years following insertion, but evidence supports use for 7 years (Thonneau, 2008). Liletta and Skyla are currently approved for 3 years. In addition to having a lower dose of progestin, Skyla is also marginally smaller in size. Mirena and Liletta have a length of 32 mm and a width of 32 mm, but with Skyla, these same dimensions measure 28 mm.
There are several progestin-mediated mechanisms by which LNG-IUS may prevent pregnancy. The progestin renders the endometrium atrophic; it stimulates thick cervical mucus that blocks sperm penetration into the uterine cavity; and it may decrease tubal motility, thereby preventing ovum and sperm union. The progestin may also inhibit ovulation, but this is not consistent (Nilsson, 1984).
Shown in Table 5-4 are the manufacturer’s contraindications to use of LNG-IUS. Women who have had a previous ectopic pregnancy may be at increased risk for another because of diminished tubal motility from progestin action. In women with uterine leiomyomas, placement of the LNG-IUS may be problematic if the uterine cavity is distorted. In their metaanalysis, Zapata and associates (2010) reported the expulsion rate to be approximately 10 percent in women with coexistent leiomyomas. However, in affected women who retained the device, menstrual blood loss will be lessened in most.
| ParaGard T 380 |
| Pregnancy or suspicion of pregnancy Uterine abnormality with distorted uterine cavity Acute PID, or current behavior suggesting a high risk for PID Postpartum or postabortal endometritis in last 3 months Known or suspected uterine or cervical malignancy Genital bleeding of unknown etiology Mucopurulent cervicitis Wilson disease Allergy to any component of ParaGard A previously placed IUD that has not been removed |
| Mirena, Liletta, and Skyla |
| Pregnancy or suspicion of pregnancy Uterine abnormality with distorted uterine cavity Use for postcoital contraception Acute PID or history of, unless there has been a subsequent intrauterine pregnancy Postpartum endometritis or infected abortion in the past 3 months Known or suspected uterine or cervical neoplasia Uterine bleeding of unknown etiology Untreated acute cervicitis or vaginitis or other lower genital tract infections Acute liver disease or liver tumor (benign or malignant) Increased susceptibility to pelvic infection A previously placed IUD that has not been removed Hypersensitivity to any component of the device Known or suspected breast cancer or other progestin-sensitive cancer |
Marketed as ParaGard, this device is composed of a stem wrapped with 314 mm2 of fine copper wire, and each arm has a 33-mm2 copper bracelet—the sum of these is 380 mm2 of copper. As shown in Figure 5-3, two strings extend from the base of the stem. The Cu-T 380A is approved for 10 years of continuous use, although it has been shown to prevent pregnancy with continuous use for up to 20 years (Bahamondes, 2005).
The intense local inflammatory response induced in the uterus by copper-containing devices leads to lysosomal activation and other inflammatory actions that are spermicidal (Alvarez, 1988; Ortiz, 1987). In the unlikely event that fertilization does occur, the same inflammatory actions are directed against the blastocyst. And finally, the endometrium becomes hostile for implantation.
During the modern renaissance of IUC, several improvements have resulted in safer and more effective models. That said, there are still some unwanted side effects and misconceptions surrounding their use.
First, fear of IUD-associated infections precluded use in the past by young women and those of low parity. Improved device design has mitigated these concerns appreciably. In addition, several well-designed studies have shown that sexual behavior and STDs are important risk factors.
With current devices, insertion generally does not increase the risk for pelvic infection. There is no evidence that prophylactic antibiotics are necessary with insertion for women at low risk for STDs (American College of Obstetricians and Gynecologists, 2014b; Walsh, 1998). Of the less than 1 in 100 women who develop an infection within 20 days of IUD insertion, most have a concomitant unrecognized cervical infection. Accordingly, women at higher risk for sexually transmitted lower genital tract infections are screened either before or at the time of IUD insertion (Centers for Disease Control and Prevention, 2015; Faúndes, 1998; Grimes, 2000). Alternatively, a small number of pelvic infections are presumed to be caused by intrauterine contamination with normal flora at the time of insertion. Thus, antibiotics selected for treatment of any pelvic infection within the early weeks following IUD insertion should be broad-spectrum to adequately cover all these organisms.
Long-term IUC use is not associated with an increased pelvic infection rate in women at low risk for STDs. Indeed, these long-term users have a pelvic infection rate comparable with that of COC users. Any pelvic infection after 45 to 60 days is considered sexually transmitted and appropriately treated as described in Chapter 3. For women who develop an infection associated with an IUD, evidence is insufficient to recommend device removal, although this is commonly done. However, close clinical reevaluation is warranted if an IUD remains (Centers for Disease Control and Prevention, 2015). In women who develop a tuboovarian abscess, the device is removed immediately after parenteral antibiotic therapy is begun.
Special concerns have arisen for women in whom Actinomyces species are identified in the lower genital tract, most commonly during Pap smear cytology reporting. Fiorino (1996) noted a 7-percent incidence in the Pap smears of IUD users compared with a 1-percent incidence in nonusers. Symptomatic pelvic actinomycosis is rare but tends to be indolent and severe.
Currently, in the absence of symptoms, incidental identification of Actinomyces species in cytologic specimens has uncertain significance. Treatment options reviewed by the American College of Obstetricians and Gynecologists (2013b) include: expectant management, an extended course of antibiotics, IUD removal, or antibiotics plus IUD removal. For women with symptomatic infection, the IUD is removed and intensive antibiotic therapy given. Actinomyces is susceptible to antibiotics with gram-positive coverage, notably the penicillins.
Nulliparous IUD candidates were previously precluded from IUC use because of fears of pelvic infection and induced sterility. Current studies indicate that the pelvic infection rate is not different from that discussed earlier (Lee, 1998; Society of Family Planning, 2010). Moreover, expulsion rates in nulliparas are similar to those in multiparas. A higher proportion of nulliparas will request removal of the device because of pain or bleeding, but overall, this population reports high levels of satisfaction with IUC. Specifically, after the first year, 75 to 90 percent continue use. Revised labeling now places no restrictions on IUC use based on parity. In addition, for the same reasons, adolescent IUD candidates may also appropriately select IUC (American College of Obstetricians and Gynecologists, 2014a). Counseling includes clear explanations of the anticipated periprocedural cramping and discomfort.
Intrauterine contraception is appropriate for affected women who are otherwise IUC candidates. Neither device type is associated with higher IUD complication rates if used in this population. Moreover, IUDs do not appear to adversely affect viral shedding or antiretroviral therapy efficacy (American College of Obstetricians and Gynecologists, 2012a).
An ideal time to improve successful provision of contraception is immediately following abortion or delivery. For women with an induced or spontaneous first- or second-trimester abortion, IUC can be placed immediately after uterine evacuation.
Insertion techniques depend upon uterine size. After first-trimester evacuation, the uterine cavity length seldom exceeds 12 cm. In these instances, the IUD can be placed using the inserter provided in the package. If the uterine cavity is larger, the IUD can be placed using ring forceps with sonographic guidance. In women for whom an IUD is placed immediately after induced abortion, the repeat induced abortion rate is only one third of the rate of women not choosing immediate IUD placement (Goodman, 2008; Heikinheimo, 2008). As perhaps expected, the risk of IUC expulsion is slightly higher when placed immediately after abortion or miscarriage, but the advantages of preventing unplanned pregnancies seem to outweigh this (Bednarek, 2011; Fox, 2011; Okusanya, 2014).
Insertion of an IUD immediately following delivery at or near term has also been studied. Placement by hand or by using an instrument has a similar expulsion rate (Grimes, 2010). As with postabortion insertion, expulsion rates by 6 months are higher than those in women whose IUD is placed after complete uterine involution. In one study, the expulsion rate in the former group was nearly 25 percent (Chen, 2010). Even in these circumstances, however, immediate placement may be beneficial because in some populations up to 40 percent of women do not return for a postpartum clinic visit (Ogburn, 2005). Finally, postpartum placement is judged to be category 1 or 2 by the US MEC, that is, its advantages consistently outweigh the risks if puerperal infection is absent (see Table 5-3).
Despite these findings, many choose to delay insertion for several weeks postpartum. Insertion at 2 weeks is quite satisfactory, and in the Parkland System Family Planning Clinics, insertion is scheduled at 6 weeks postpartum to ensure complete uterine involution.
Commonly, IUC may be associated with changes in menstrual patterns. Women who choose the Cu-T 380A are informed that increased dysmenorrhea and bleeding with menses may develop. Objectively, no clinically significant hemoglobin changes are generally expected (Tepper, 2013). Treatment with a nonsteroidal antiinflammatory drug (NSAID) will usually diminish the amount of bleeding—even normal amounts—and also relieve dysmenorrhea (Grimes, 2006).
With the LNG-IUS, women are counseled to expect irregular spotting for up to 6 months after insertion and thereafter to expect monthly menses to be lighter or even absent. Specifically, the Mirena device is associated with progressive amenorrhea, which is reported by 30 percent of women after 2 years and by 60 percent after 12 years (Ronnerdag, 1999). As noted in Chapter 8, the LNG-IUS device reduces menstrual blood loss and is an effective treatment for some women with heavy menstrual bleeding (American College of Obstetricians and Gynecologists, 2014e). This is often associated with improved dysmenorrhea.
Approximately 5 percent of women will spontaneously expel their IUD during the first year of use. This is most likely during the first month. Accordingly, a woman is instructed to periodically palpate the marker strings protruding from the cervical os. This can be accomplished by either sitting on the edge of a chair or squatting down and then advancing the middle finger into the vagina until the cervix is reached. Following insertion of either IUD type, women are reappointed for a visit within several weeks, usually after completion of menses. At this meeting, any side effects are addressed, and IUD placement is confirmed by visualizing the marker strings. Some recommend barrier contraception to ensure contraception during this first month. This may be especially desirable if a device has been expelled previously.
The uterus may be perforated either with a uterine sound or with an IUD. Perforations may be clinically apparent or silent. Their frequency depends on operator skill and is estimated to be approximately 1 per 1000 insertions (World Health Organization, 1987). In some cases, a partial perforation at insertion is followed by migration of the device completely through the uterine wall. Occasionally, perforation occurs spontaneously.
In some cases, the IUD marker strings may not be palpated or seen during speculum examination. During the investigation, a nonpregnant patient should use alternative contraception. Possibilities include that the device was expelled silently, the device has partially or completely perforated the uterus, the woman is pregnant and the enlarging uterus has drawn the device upward, or the marker strings are temporarily hidden within the endocervical canal. An IUD should not be considered expelled unless it was seen by the patient.
Initially, an endocervical brush or similar instrument can be used to gently draw the string out of the cervical canal. If this is unsuccessful, then at least two options are available. After pregnancy has been excluded, the uterine cavity is gently probed using an instrument such as Randall stone forceps or a rod with a hooked end. The strings or device will often be found with this method. If not successful, at this juncture, or possibly as a first choice, transvaginal sonography (TVS) is performed. As described in Chapter 2, 3-dimensional TVS offers improved visualization (Moschos, 2011). If the device is not seen within either the uterine cavity or uterine walls, then an abdominal radiograph, with or without a uterine sound in place, may localize it. Another option includes hysteroscopy.
Management decisions depend upon where the device is located and whether there is a coexistent intrauterine pregnancy. First, a device may penetrate the uterine wall in varying degrees. It should be removed, and this approach varies by IUD location. Devices with a predominantly intrauterine location are typically managed by hysteroscopic IUD removal. In contrast, devices that have nearly completely perforated through the uterine wall are more easily removed laparoscopically.
For women with an intraabdominal IUD, an inert-material device located outside the uterus may cause harm, but not universally. Bowel perforations—both large and small—as well as bowel fistulas have been reported. Once identified laparoscopically, these inert devices can easily be retrieved via laparoscopy or less commonly by colpotomy. Conversely, an extrauterine copper-bearing device induces an intense local inflammatory reaction with adhesions. Thus, they are more firmly adhered, and laparotomy may become necessary (Balci, 2010).
In those with pregnancy and an IUD, early pregnancy identification is important. Up to approximately 14 weeks’ gestation, the IUD strings may be visible within the cervix, and if seen, they are grasped to remove the entire IUD. This action reduces subsequent complications such as late abortion, sepsis, and preterm birth (Alvior, 1973). Tatum and colleagues (1976) reported an abortion rate of 54 percent with the device left in place compared with a rate of 25 percent if it was promptly removed. More recently, a study from Israel by Ganer and coworkers (2009) reported pregnancy outcomes from 1988 to 2007 in 292 women who conceived with a Cu-IUD in place. Outcomes were compared in the two groups of women with and without IUD removal as well as with the general obstetrical population. As shown in Table 5-5, in general, the group of women with an IUD left in place had the worst outcomes. Importantly, however, the group in whom the IUD was removed still had significantly worse outcomes compared with those of the general population. Of special note, Vessey and associates (1979) had previously reported that fetal malformations were not increased in pregnancies in which the device was left in place. In the Ganer study, it is particularly worrisome that this rate was doubled compared with women in whom the device was removed. The distribution of malformations was notable in that 12 percent were skeletal malformations. In contrast, there were no chromosomal anomalies identified in fetuses born to women from the two IUD groups.
| Outcomea | IUD in situ(n = 98) | IUD removed (n = 194) | No IUD (n = 141,191) | p value |
| PROM | 10.2 | 7.7 | 5.7 | .021 |
| Preterm delivery | 18.4 | 14.4 | 7.3 | <.001 |
| Chorioamnionitis | 7.1 | 4.1 | 0.7 | <.001 |
| Fetal growth restriction | 1.0 | 0.5 | 1.7 | NS |
| Abruption | 4.1 | 2.1 | 0.7 | <.001 |
| Previa | 4.1 | 0.5 | 0.5 | <.001 |
| Cesarean | 32 | 21 | 13 | <.001 |
| Low birthweight <2500 g <1500 g | 11.2 5.1 | 13.4 3.6 | 6.7 1.1 | <.001 <.001 |
| Perinatal death | 1.0 | 1.5 | 1.2 | NS |
| Malformations | 10.2 | 5.7 | 5.1 | <.041 |
Because of these findings, if pregnancy continuation is desired, it is recommended that with early pregnancies the IUD be removed. However, if the strings are not visible, attempts to locate and remove the device may result in pregnancy loss. This risk must be weighed against the risk of leaving the device in place. If removal is attempted, TVS can be used. If attempts at removal are followed by evidence for infection, then antimicrobial treatment is begun and is followed by prompt uterine evacuation.
The risk of an associated ectopic pregnancy has been clarified over the past few years. IUC is effective in preventing all pregnancies. Specifically, the contraceptive effect of IUC decreases the absolute number of ectopic pregnancies by half compared with the rate in women who do not use contraception (World Health Organization, 1985, 1987). However, the IUC mechanisms of action are more effective in preventing intrauterine implantation. Thus, if IUC fails, a higher proportion of pregnancies are likely to be ectopic (Furlong, 2002).
Before IUD insertion, the FDA requires that a woman be given a brochure detailing the side effects and apparent risks from its use. Timing of insertion influences the ease of placement as well as pregnancy and expulsion rates. When done toward the end of normal menstruation, when the cervix is usually softer and somewhat more dilated, insertion may be easier, and early pregnancy can be excluded. However, insertion is not limited to this time. For a woman who is sure she is not pregnant and does not want to be pregnant, insertion may be carried out any time during the menstrual cycle. Insertion immediately postpartum or postabortion is also feasible and discussed on page 110.
Prior to insertion, a pelvic examination is completed to identify uterine position and size. Abnormalities are evaluated as they may contraindicate the device. Evidence for infection such as a mucopurulent discharge or significant vaginitis is appropriately treated and resolved before insertion.
For pain management, the most effective method of analgesia has not been established, and patient and provider preference directs selection. Options include NSAIDs, topical lidocaine, or paracervical block. Misoprostol is thought to advance cervical softening to mitigate cervical dilatation pain. However, few studies have adequately evaluated these (Allen, 2009).
At the beginning of the insertion procedure, the cervical surface is cleaned with an antiseptic solution, and a tenaculum is placed on the cervical lip. The uterus is sounded to guide correct depth placement. Specific steps for IUD insertion are outlined and illustrated in Figs. 5-4 and 5-5. During insertion, if there is concern for correct IUD positioning, then placement may be checked by inspection or by sonography. If not positioned completely within the uterus, the device is removed and replaced with a new one. An expelled or partially expelled device should not be reinserted.
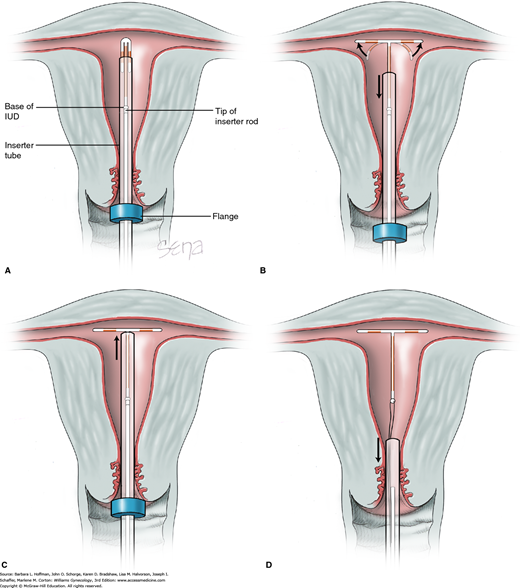
FIGURE 5-4
Insertion of ParaGard T 380A. The IUD is loaded into its inserter tube not more than 5 minutes before insertion. If longer, the malleable arms can retain “memory” of the inserter and remain bent inward. A blue plastic flange on the outside of the inserter tube is positioned from the IUD tip to reflect the uterine depth ascertained during sounding. The IUD arms should lie in the same plane as the flat portion of the blue flange. A. The inserter tube, with the IUD loaded, is passed into the endometrial cavity. When the blue flange abuts the cervix, insertion stops. B. To release the IUD arms, the solid white rod within the inserter tube is held steady while the inserter tube is withdrawn no more than 1 cm. C. The inserter tube is then carefully moved upward toward the top of the uterus until slight resistance is felt. D. First, the solid white rod and then the inserter tube are withdrawn individually. At completion, only the threads are visible protruding from the cervix. These are trimmed to allow 3 to 4 cm to extend into the vagina.
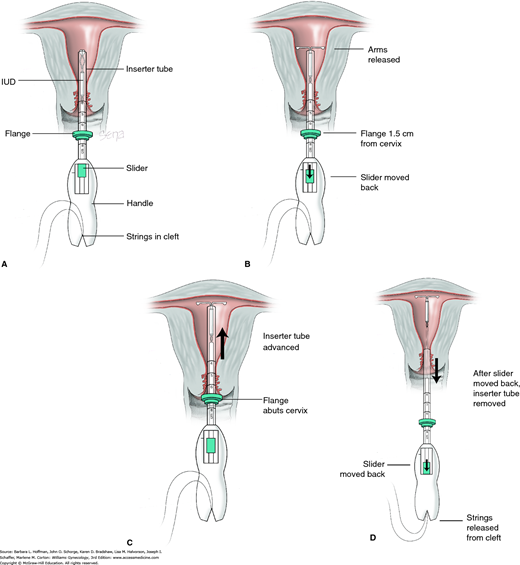
FIGURE 5-5
Insertion of Mirena intrauterine system. Threads from behind the slider are first released to hang freely. The teal-colored slider found on the handle should be positioned at the top of the handle nearest the device. The IUD arms are oriented horizontally. A. As both free threads are pulled outward, the Mirena IUD is drawn into the inserter tube. The threads are then moved upward from below and tightly fixed into the handle’s cleft. A flange on the outside of the inserter tube is positioned from the IUD tip to reflect the depth found with uterine sounding. B. While inserting the Mirena device, the slider is held firmly in position at the top of the handle. Gentle traction is created by outward traction on the tenaculum to align the cervical canal with the uterine cavity. The inserter tube is gently threaded into the uterus until the flange lies 1.5 to 2 cm from the external cervical os to allow the arms to open. While holding the inserter steady, the IUD arms are released by pulling the slider back only to the raised horizontal line on the handle. This position is held for 15 to 20 seconds to allow the arms to fully open. C. The inserter is then gently guided into the uterine cavity until its flange touches the cervix. D. The device is released by holding the inserter firmly in position and pulling the slider back all the way. The threads will be released automatically. The inserter may then be removed. IUD strings are trimmed to leave approximately 3 cm visible outside the cervix.
Contraception can be provided by a progestin-containing device that is implanted subdermally and releases hormone over many years. The devices are coated with a polymer to prevent fibrosis. Several systems have been developed, but only one is available in the United States. The initial implant, the Norplant System, releases levonorgestrel from six Silastic rods. It was withdrawn from the U.S. market, and a fund has been established by the manufacturer to ensure access to patients for removal. Supposedly, the silicone-based rods caused ill-defined symptoms that were reversed with removal. A newer two-rod levonorgestrel system, Jadelle, has received FDA approval but is not marketed or distributed in the United States (Sivin, 2002). Sino-implant II is a structurally and pharmacologically similar system to Jadelle. It is manufactured in China and approved for use by several countries in Asia and Africa (Steiner, 2010).
The implant Nexplanon is currently the only subdermal contraceptive implant marketed in the United States. It is a single-rod subdermal implant containing 68 mg of a progestin—etonogestrel—and covered by an ethylene vinyl acetate copolymer. Nexplanon has replaced the earlier etonogestrel implant, Implanon.
For Nexplanon, contraception is provided by progestin released continuously to suppress ovulation, increase cervical mucus viscosity, and induce endometrial atrophy. The etonogestrel implant will provide contraception for up to 3 years. At this time, the device is removed, and another rod may be placed within the same incision site. Contraindications for this device are similar to those cited for other progestin-containing methods. Specifically, these include pregnancy, thrombosis or thromboembolic disorders, benign or malignant hepatic tumors, active liver disease, undiagnosed abnormal genital bleeding, or breast cancer (Merck, 2014). Importantly, patients are counseled that Nexplanon causes irregular bleeding that does not normalize over time. Thus, women who cannot tolerate unpredictable and irregular spotting or bleeding should select an alternative method.
Nexplanon is inserted subdermally along the biceps groove of the inner arm and 6 to 8 cm from the elbow (Fig. 5-6). Immediately following insertion, the provider and patient should document that the device is palpable beneath the skin. When Nexplanon is removed, this superficial location allows in-office extraction of the implant. Through a small incision large enough to admit hemostat tips, the implant is grasped and removed. If desired, a new rod can be placed through this same incision.
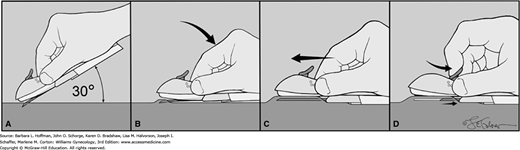
FIGURE 5-6
Nexplanon insertion. A sterile pen marks the insertion site, which is 8 to 10 cm proximal to the medial humeral condyle. A second mark is placed 4 cm proximally along the arm’s long axis. The area is cleaned aseptically, and a 1-percent lidocaine anesthetic track is injected along the planned insertion path. A. The insertion device is grasped at its gripper bubbles found on either side, and the needle cap is removed outward. The device can be seen within the needle bore. The needle bevel then pierces the skin at a 30-degree angle. B. Once the complete bevel is subcutaneous, the needle is immediately angled downward to lie horizontally. C. Importantly, the skin is tented upward by the needle as the needle is slowly advanced horizontally and subdermally. D. Once the needle is completely inserted, the lever on the top of the device is pulled backward toward the operator. This retracts the needle and thereby deposits the implant. The device is then lifted away from the skin. After placement, both patient and operator should palpate the 4-cm implant.
If Nexplanon is not palpable, it can be imaged by radiography, computed tomography (CT), sonography, or magnetic resonance (MR) imaging. Norplant and Jadelle are also radiopaque. This is an advantage compared with Implanon, which is not radiopaque and requires sonography with a 10- to 15-MHz sonographic transducer or MR imaging for identification (Shulman, 2006). In the rare event that an etonogestrel implant cannot be palpated or identified radiologically, the manufacturer can be contacted and arrangements made for etonogestrel level measurement (Merck, 2014).
In 2011 to 2013, surgical sterilization was one of the most commonly reported forms of contraception in childbearing-aged women in the United States (Daniels, 2014). These procedures cannot be tracked accurately because most interval tubal sterilizations and vasectomies are performed in ambulatory surgical centers. However, according to the National Survey of Family Growth, approximately 643,000 female tubal sterilizations are performed annually in the United States (Chan, 2010). The two most commonly employed forms in this country are bilateral tubal ligation—frequently via laparoscopy—and hysteroscopic tubal sterilization. The latter has become popular, and in some settings, it is used in up to half of nonpuerperal female sterilizations (Shavell, 2009).
Over the past 20 years, several important multicenter studies regarding sterilization have been performed by investigators of the Collaborative Review of Sterilization (CREST) and the Centers for Disease Control and Prevention. Data from many of these studies are subsequently described.
This is usually accomplished by occlusion or division of the fallopian tubes to prevent ovum passage and fertilization. According to the National Health Statistics Report, 27 percent of contracepting women in the United States use this method (Jones, 2012). Approximately half of tubal sterilization procedures are performed in conjunction with cesarean delivery or soon after vaginal delivery (MacKay, 2001). Accordingly, this is termed puerperal sterilization. The other half of tubal sterilization procedures are done at a time unrelated to recent pregnancy, that is, nonpuerperal tubal sterilization. This is also termed interval sterilization. In most instances, nonpuerperal tubal sterilization is accomplished via laparoscopy or hysteroscopy.
There are three methods, along with their modifications, that are used for tubal interruption. These include application of various permanent rings or clips to the fallopian tubes; electrocoagulation of a tubal segment; or ligation with suture material, with or without removal of a tubal segment. In a Cochrane review, Lawrie and colleagues (2011) concluded that all of these are effective in preventing pregnancy.
Electrocoagulation is used for destruction of a segment of tube and can be accomplished with either unipolar or bipolar current. Although unipolar coagulation has the lowest long-term failure rate, it also has the highest serious complication rate. For this reason, bipolar coagulation is favored by most (American College of Obstetricians and Gynecologists, 2013a).
Mechanical methods of tubal occlusion can be accomplished with: (1) a silicone rubber band such as the Falope Ring or the Tubal Ring, (2) the spring-loaded Hulka-Clemens clip—also known as the Wolf clip, or (3) the silicone-lined titanium Filshie clip. The steps to these procedures are described in Section 44-2 of the surgical atlas. In a randomized trial of 2746 women, Sokal and associates (2000) compared the Tubal Ring and Filshie clip and reported similar rates of safety and 1-year pregnancy rates of 1.7 per 1000 women. All of these mechanical occlusion methods have favorable long-term success rate.
Suture ligation with tubal segment excision is more often used for puerperal sterilization. Methods include Parkland, Pomeroy, and modified Pomeroy, which are illustrated in Section 43-7.
The type of abdominal entry for sterilization is also variable. Laparoscopic tubal ligation is the leading method used in this country for nonpuerperal female sterilization (American College of Obstetrics and Gynecologists, 2013a). This is frequently done in an ambulatory surgical setting under general anesthesia, and the woman can be discharged several hours later. Alternatively, some choose minilaparotomy using a 3-cm suprapubic incision. This is especially popular in resource-poor countries. With either laparoscopy or minilaparotomy, major morbidity is rare. Minor morbidity, however, was twice as common with minilaparotomy in a review by Kulier and associates (2004). Finally, the peritoneal cavity can also be entered by colpotomy through the posterior vaginal fornix, although this approach is infrequently used.
Indications for this elective procedure for sterilization include a request for sterilization with clear understanding that this is permanent and irreversible. Each woman is counseled regarding all alternative contraceptive options and their efficacy. Each woman is also informed regarding her sterilization options, which include laparoscopic or hysteroscopic tubal occlusion or bilateral total salpingectomy. The risks and benefits of each are thoroughly discussed. Many women may also have questions or misunderstanding about possible long-term outcomes after female sterilization. As with any operation, surgical risks are assessed, and occasionally the procedure may be contraindicated.
The Society of Gynecologic Oncology (2013) currently recommends consideration of bilateral total salpingectomy as a preventive measure against serous ovarian and peritoneal cancers. As discussed in Chapter 35, this may be especially relevant for women at greatest risk for these cancers, namely, women with BRCA1 or BRCA2 mutation. Most pelvic serous cancers are thought to originate in the distal fallopian tube. Total salpingectomy may confer up to a 34-percent reduction in endometrioid and serous ovarian cancer rates (Erickson, 2013; Sieh, 2013). If risk-reducing salpingectomy is elected in women with BRCA mutations, the pathology requisition form should state this genetic information. This prompts more thorough tubal specimen sectioning to search for cancer and precancerous lesions, which can be found in the tubes of BRCA mutation carriers.
In low-risk women, because the ovarian cancer risk is less than 2 percent, risk-reducing salpingectomy as an isolated procedure is likely unwarranted. However, if surgery such as hysterectomy or tubal sterilization is planned, women are counseled regarding the risks and benefits of complete fallopian tube excision (Anderson, 2013). As advantages, total salpingectomy may decrease risks for subsequent tubal surgery. As disadvantages, operating time may be increased by 10 minutes, and more importantly, the degree of long-term ovarian blood supply disruption with total salpingectomy is not clearly defined (Creinin, 2014).
Invariably, some women will later express regrets about sterilization. From a CREST study, Jamieson and coworkers (2002) reported that by 5 years, 7 percent of women undergoing tubal ligation had regrets. This is not limited to female sterilization, as 6 percent of women whose husbands had undergone vasectomy had similar remorse. The cumulative probability of regret within 14 years of sterilization was 20 percent for women aged 30 or younger at sterilization compared with only 6 percent for those older than 30 years (Hillis, 1999).
No woman should undergo tubal sterilization believing that subsequent fertility is guaranteed either by surgical reanastomosis or by assisted reproductive techniques. These are technically difficult, expensive, and not always successful. Pregnancy rates vary greatly depending upon age, the amount of tube remaining, and the technology used. Pregnancy rates range from 50 to 90 percent with surgical reversal (Deffieux, 2011). Of note, pregnancies that result after tubal sterilization reanastomosis are at risk to be ectopic.
Reasons for interval tubal sterilization failure are not always apparent, but some have been identified. First, surgical error may occur and likely accounts for 30 to 50 percent of cases. Second, tubal fistula may complicate occlusion methods. Although usually encountered with electrocoagulation procedures, fistulas from inadequate or defective electric current delivery are now less likely because an amp meter is used routinely. In some cases, sterilization failure may follow spontaneous reanastomosis of the tubal segments. With faulty clips, occlusion can be incomplete. Last, luteal phase pregnancy may occur and describes the situation in which a woman is already pregnant when the procedure is performed. This can often be avoided by scheduling surgery during the menstrual cycle’s follicular phase and by preoperative human chorionic gonadotropin (hCG) testing.
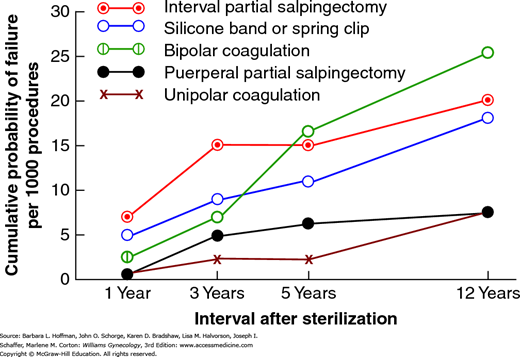
The overall failure rate reported from the CREST studies was 1.3 percent of 10,685 tubal sterilization surgeries. As shown in Figure 5-7, these rates vary for different procedures. And even with the same operation, failure rates vary. For example, with electrocoagulation, if fewer than three tubal sites are coagulated, the 5-year cumulative pregnancy rate approximates 12 per 1000 procedures. However, it is only 3 per 1000 if three or more sites are coagulated (Peterson, 1999). The lifetime increased cumulative failure rates over time are supportive that failures after 1 year are not likely due to technical errors. Indeed, Soderstrom (1985) found that most sterilization failures were not preventable.
FIGURE 5-7
Data from the U.S. Collaborative Review of Sterilization (CREST) shows the cumulative probability of pregnancy per 1000 procedures by five methods of tubal sterilization. (Data from Peterson HB, Xia Z, Hughes JM, et al: The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization. Am J Obstet Gynecol 174(4):1161, 1996.)
With method failure, pregnancies following tubal sterilization have a high incidence of being ectopically implanted compared with the rate in a general gynecologic population. These rates are especially high following electrocoagulation procedures, in which up to 65 percent of pregnancies are ectopic. With failures following other methods—ring, clip, tubal resection—this percentage is only 10 percent (Peterson, 1999). Importantly, ectopic pregnancy must be excluded when any symptoms of pregnancy develop in a woman who has undergone tubal sterilization.
Several studies have evaluated the risk of heavy menstrual bleeding and intermenstrual bleeding following tubal sterilization, and many report no link (DeStefano, 1985; Shy, 1992). In addition, Peterson and coworkers (2000) compared long-term outcomes of 9514 women who had undergone tubal sterilization with a cohort of 573 women whose partners had undergone vasectomy. Risks for heavy menstrual bleeding, intermenstrual bleeding, and dysmenorrhea were similar in each group. Perhaps unexpectedly, women who had undergone sterilization had decreased duration and volume of menstrual flow, they reported less dysmenorrhea, but they had an increased incidence of cycle irregularity.
Other long-term effects have also been studied. It is controversial whether risks for subsequent hysterectomy are increased (Pati, 2000). In a CREST surveillance study, Hillis and associates (1997) reported that 17 percent of women undergoing tubal sterilization subsequently had undergone hysterectomy by 14 years. Although they did not compare this incidence with a control cohort, the indications for hysterectomy were similar to those for nonsterilized women who had undergone a hysterectomy. Women are highly unlikely to develop salpingitis following sterilization (Levgur, 2000). Tubal sterilization appears to have a protective effect against ovarian cancer, but not breast cancer (Westhoff, 2000).
Some psychological sequelae of sterilization were evaluated in a CREST study by Costello and associates (2002). These investigators reported that tubal ligation did not change sexual interest or pleasure in 80 percent of women. In the remaining 20 percent of women who reported a change, 80 percent described the changes to be positive.
Various methods of sterilization can be completed using a transcervical approach to reach the tubal ostia. Within each ostium, occlusion is achieved by placing either mechanical devices or chemical compounds.
Mechanical methods employ insertion of a device into the proximal fallopian tubes via hysteroscopy. One system, Essure, is FDA-approved for use in the United States. A second system, Adiana Permanent Contraception, was taken off the market in 2013.
The Essure Permanent Birth Control System consists of a microinsert made of a stainless steel inner coil that is enclosed in polyester fibers. These fibers are surrounded by an expandable outer coil made of nitinol—a nickel and titanium alloy used in coronary artery stents (Fig. 5-8). Fibroblastic proliferation within the fibers causes tubal occlusion. The Essure technique is described in Section 44-16. Analgesia provided by intravenous sedation or paracervical block will successfully alleviate pain (Cooper, 2003). In some women, general anesthesia is preferred.
By far, the overwhelming advantage of hysteroscopic sterilization is that it can be performed in the office. In addition, the procedure times average less than 20 minutes. Abnormal anatomy may preclude procedure completion. One year after placement, Essure contraceptive failure rates range from less than 1 percent to 5 percent (Gariepy, 2014; Munro, 2014).
Three months following device insertion, hysterosalpingography (HSG) is required to confirm complete occlusion (American College of Obstetricians and Gynecologists, 2012b). Prior to undergoing the procedure, patients are counseled on the importance of HSG compliance as up to half of all unplanned pregnancies after hysteroscopic sterilization may be associated with follow-up noncompliance (Cleary, 2013; Levy, 2007). Other reasons for subsequent unplanned pregnancy include incomplete occlusion (10 percent), incorrect HSG interpretation (33 percent), and an established pregnancy prior to the procedure (1 percent) (Jost, 2013; Munro, 2014). In some women, occlusion is incomplete at 3 months, and the study is then repeated at 6 months postoperatively. Until tubal occlusion is established, another method of contraception is needed. Transvaginal sonography has been investigated as an alternative confirmation tool, but currently HSG is required by the FDA (Veersema, 2011).
Pelvic pain after hysteroscopic sterilization is uncommon. If pelvic pain presents soon after the procedure, symptoms are likely to resolve by 3 months postoperatively, around the same time as the follow-up HSG (Arjona Berral, 2014; Yunker, 2015).
As with all sterilization procedures, Essure placement should be considered permanent. The success rate of subsequent spontaneous pregnancy after microsurgery tubal reversal ranges between 0 and 36 percent (Fernandez, 2014; Monteith, 2014).
Agents may be placed into the uterine cavity or tubal ostia to incite an inflammatory response to cause tubal occlusion. A method that has been used worldwide in more than 100,000 women consists of using an IUD-type inserter to place quinacrine pellets into the uterine fundus. It is effective, especially considering its simplicity. Pregnancy rates reported by Sokal and colleagues (2008) were 1 and 12 percent at 1 and 10 years, respectively. Although the WHO recommends against its use because of carcinogenesis concerns, it remains an important method for resource-poor countries (Castaño, 2010; Lippes, 2002).
For a woman with uterine or other pelvic disease for which hysterectomy may be indicated, this may be the ideal form of sterilization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree