BIOLOGY OF CANCER GROWTH
In principle, chemotherapeutic drugs are able to treat cancer and spare normal cells by exploiting inherent differences in their individual growth patterns. Each tumor type has its own characteristics, which explain why the same chemotherapy regimen is not equally effective for the whole spectrum of gynecologic cancers. Selecting appropriate drugs and limiting toxicity demands an understanding of cellular kinetics and biochemistry.
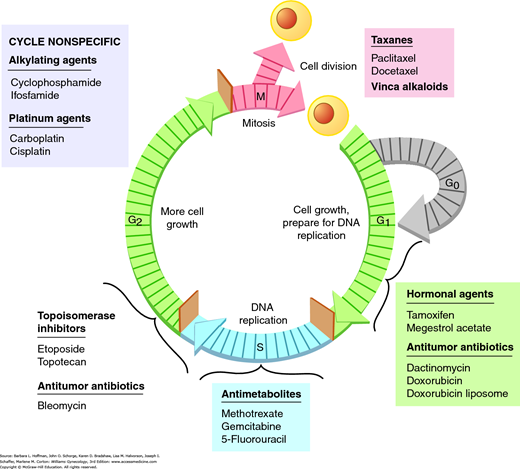
All dividing cells follow the same basic sequence for replication. The cell generation time is the time required to complete the five phases of the cell cycle (Fig. 27-1). The G1 phase (G = gap) involves various cellular activities, such as protein synthesis, RNA synthesis, and DNA repair. When prolonged, the cell is considered to be in the G0 phase, that is, the resting phase. G1 cells may either terminally differentiate into the G0 phase or reenter the cell cycle after a period of quiescence. During the S phase, new DNA is synthesized. The G2 (premitotic) phase is characterized by cells having twice the DNA content as they prepare for division. Finally, actual mitosis and chromosomal division takes place during the M phase.
Tumors do not typically have faster generation times. They instead have many more cells in the active phases of replication and have dysfunctional apoptosis (programmed cell death), hence proliferation. In contrast, normal tissues have a much larger number of cells in the G0 phase. As a result, cancer cells proceeding through the cell cycle may be more sensitive to chemotherapeutic agents, whereas normal cells in G0 are protected. This growth pattern disparity underlies the effectiveness of chemotherapeutic agents.
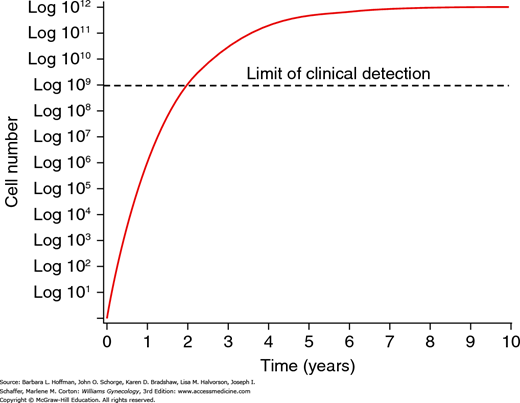
Tumors are characterized by a gompertzian growth pattern (Fig. 27-2). Fundamentally, a tumor mass requires progressively longer times to double in size as it enlarges. When a cancer is microscopic and nonpalpable, growth is exponential. However, as a tumor enlarges, the number of its cells undergoing replication decreases due to limitations in blood supply and increasing interstitial pressure.
When tumors are in the exponential phase of gompertzian growth, they should be more sensitive to chemotherapy because a larger percentage of cells are in the active phase of the cell cycle. For this reason, metastases should be more sensitive to chemotherapy than the primary tumor. To capitalize on this potential benefit, advanced ovarian cancer is usually first treated with surgery to remove the primary tumor, debulk large masses, and leave only microscopic residual disease for the adjuvant chemotherapy to act on. In addition, when a tumor mass shrinks in response to treatment, the presumption is that a greater number of cells will enter the active phase of the cell cycle to accelerate growth. This larger percentage of replicating cells should also increase the sensitivity of a tumor to chemotherapy.
The time needed for a tumor to double in size is commonly referred to as its doubling time. Whereas the cell cycle generally refers to the activity of individual tumor cells, doubling time refers to the growth of an entire heterogeneous tumor mass. In humans, the doubling times of specific tumors vary greatly. The speed with which tumors grow and double in size is largely regulated by the number of cells that are actively dividing—known as the growth fraction. Typically, only a small percentage of the tumor will have cells that are rapidly proliferating. The remaining cells are in the G0 resting phase. In general, tumors that are cured by chemotherapy are those with a high growth fraction, such as gestational trophoblastic neoplasia. When tumor volume is reduced by surgery or chemotherapy, the remaining tumor cells are theoretically propelled from the G0 phase into the more vulnerable phases of the cell cycle, rendering them susceptible to chemotherapy.
Chemotherapeutic agents typically work by first-order kinetics to kill a constant fraction of cells rather than a constant number. For example, one dose of a cytotoxic drug may result in a few logs (102 to 104) of cell kill. This, however, is not curative since tumor burden may be 1012 cells or more. Thus, the magnitude of cell kill necessary to eradicate a tumor typically requires intermittent courses of chemotherapy. In general, a cancer’s curability is inversely proportional to the number of viable tumor cells at the beginning of chemotherapy.
Some drugs achieve cell kill at several phases of the cell cycle. These cell cycle-nonspecific agents act in all phases of replication from G0 to the M phase. Cell cycle-specific agents act only on cells that are in a specific phase. By combining drugs that act in different phases of the cell cycle, the overall cell kill should be enhanced.
CLINICAL USE OF CHEMOTHERAPY
Chemotherapy may be used in at least five different ways. The term induction chemotherapy is defined as primary treatment for patients with an advanced malignancy when no feasible alternative treatment exists. Adjuvant chemotherapy is given to destroy remaining microscopic cells that may be present after the primary tumor is removed by surgery. Neoadjuvant chemotherapy refers to drug treatment directed at an advanced cancer to decrease preoperatively the extent or morbidity of a subsequent surgical resection. Consolidation (or maintenance) chemotherapy is given after cancer has disappeared following the initial therapy to prolong the duration of clinical remission or to prevent ultimate relapse. Therapy applied to recurrent disease or to a tumor that is refractory to initial treatment is termed salvage (or palliative) chemotherapy. In these incurable patients, the intent is to achieve tumor shrinkage or stability but maintain quality of life.
In general, chemotherapy is used with either curative or palliative intent. When implementing chemotherapy with curative intent, the number of courses is typically predefined. Emphasis is placed on maintaining curative dosages and adhering closely to the treatment schedule. This may lead to significant toxicity and require growth-factor support. However, for the possibility of achieving cure, these side effects are typically deemed acceptable.
Chemotherapy is often not used with curative intent, and the treating clinician must balance several factors to provide effective, compassionate palliation. Thus, in this setting, greater importance is attached to avoiding excessive toxicity. In many ways, the use of chemotherapy for palliation exemplifies the “art” of medicine. Instead of a defined number of treatment courses, a clinician must frequently revisit the treatment effectiveness and alter the dosage and timing of chemotherapy administration accordingly.
With few exceptions, single drugs administered at clinically tolerable doses do not cure cancer. However, using two or more drugs simultaneously may greatly exacerbate toxicity. Thus, in principle, the goal of combination chemotherapy is to provide maximum cell kill with minimal or tolerable adverse patient side effects. Drugs are selected based on their proven efficacy as single agents, different mechanisms of action, and toxicities that overlap minimally or not at all.
Combination chemotherapy is more effective in attacking heterogeneous populations of cells. Moreover, the use of multiple drugs with differing mechanisms of action tends to minimize the emergence of drug resistance. Typically, drugs used in combination should have clinical data indicating that their effects will be synergistic or at least additive. Drugs in combination are used at their optimal doses and schedules. Dose reductions initiated solely to allow the addition of other agents are counterproductive because most drugs must be used near their maximum tolerated dose to ensure efficacy.
Frequently, chemotherapy is combined with radiation therapy or sequenced with surgery. With chemoradiation, the goal is to achieve local control by chemically rendering the tumor more sensitive to radiation. For example, care of locally advanced cervical cancer was transformed by adding weekly cisplatin to standard radiotherapy. In addition, concurrent chemotherapy is intended to treat micrometastases outside the radiation field.
However, treatment-related toxicity is also increased. Patients previously treated with radiation therapy may have bone marrow, skin, or other body systems that are more susceptible to chemotherapy toxicity. As a result, dose reductions or delays are commonplace. Furthermore, chemotherapy is generally less effective in tumors that lie within a previously irradiated field due to increased fibrosis and capillary destruction.
Combining chemotherapy with surgery has many different applications. A woman with endometrial cancer may have nodal metastases detected during surgery, and receive pelvic radiation preceded or followed by combination chemotherapy. Alternatively, a woman with recurrent ovarian cancer may be treated by combination chemotherapy with or without preceding secondary cytoreductive surgery. The purpose of sequencing treatment in this way is to reduce tumor bulk and thereby augment chemotherapy effectiveness. In general, adjunctive therapy is begun within a few weeks after surgery.
To effectively counsel a gynecologic cancer patient and then guide her chemotherapeutic treatment course requires a comprehensive understanding of the diagnosis, alternatives, and goals of care. Coexisting conditions or tumor-related complications (e.g., deep-vein thrombosis) may need to be addressed. As the intended therapy is finalized, extensive information regarding anticipated side effects is provided to allay concerns and reduce anxiety. A consent form must be reviewed and signed by the patient, in addition to clarification of all potential logistical challenges (e.g., intravenous access).
Prior to drug infusion, a complete medical history and comprehensive physical examination are mandatory. Blood work, including a complete blood count, comprehensive metabolic panel, and tumor markers (e.g., CA125) as indicated, are drawn and reviewed before orders to begin infusion are signed. Drug administration must take place in a setting where staff are immediately available to intervene should the need arise. Afterward, the patient is provided contact numbers in case of questions, problems, or other concerns that can often develop prior to the next visit.
Typically, regular office visits shortly before or on the day of treatment allow assessment of toxicity and general health. Patient examination and review of blood work results, in the context of the tumor response and overall treatment goal, will help determine whether drugs are changed or their dosages revised. Over time, the treatment strategy is continually reassessed as circumstances change.
PHARMACOLOGIC PRINCIPLES
Overall, treatment effectiveness depends on drug concentration and duration of exposure to critical tumor sites. Chemotherapeutic agents typically have a narrow therapeutic range or “window.” Thus, doses must be calculated accurately to achieve an optimal effect above a critical threshold while avoiding undue toxicity.
Most commonly, chemotherapy doses are calculated based on the patient’s body surface area (BSA) and are expressed in milligrams per meter squared (mg/m2). BSA is a better indicator of metabolic mass than body weight because it is less affected by abnormal adipose mass. This calculation ensures that each patient receives proportionally similar drug amounts. Although height is a fixed variable, patient weights are obtained prior to every therapy course, as they may fluctuate significantly. Rarely, tissue edema or ascites must be factored, since doses should be based on weight without this coexisting fluid. The BSA is most often calculated by using a nomogram (standard reference graph table). Consistent derivation of the BSA at each visit is important, and various calculators are routinely available via software or online (http://www.globalrph.com/bsa2.htm). “Normal” adult BSA for women approximates 1.7 m2.
Alternatively, the dosing of some drugs is more specific. For example, bevacizumab is a monoclonal antibody metabolized and eliminated via the reticuloendothelial system. It is dosed only by patient weight (mg/kg). For renally excreted drugs, such as carboplatin, dosing may be based on an estimate of the glomerular filtration rate (Calvert formula).
The amount of drug administered over time is known as the dose intensity (or density). Its primary importance is in highly responsive tumors, in which cure can be achieved with chemotherapy. However, in other less sensitive tumors, it may not be possible to increase the dose to a level sufficient to produce demonstrable benefit without producing dose-limiting toxicity. On the other hand, reducing dose intensity to decrease toxicity can produce inferior therapeutic results.
Chemotherapy may be administered systemically or regionally. Oral, intravenous (IV), subcutaneous (SC), or intramuscular (IM) routes comprise systemic treatment options. Regional chemotherapy is aimed at delivering drugs directly into the cavity in which the tumor is located. Clearance for many agents from a body cavity is slower than from systemic circulation. As a result, cancer cells are exposed longer to higher concentrations of active agents. This technique has been most extensively studied in ovarian cancer, in which tumors are usually confined to the intraperitoneal (IP) space. Clinical studies have uniformly demonstrated a pharmacologic advantage favoring administration into the IP compartment. However, penetration into peritoneal tumor nodules by passive diffusion is often limited by the presence of intraabdominal adhesions, poor fluid circulation, fibrotic tumor encapsulation, and coexisting ascites. Because of these limitations in drug penetration, IP chemotherapy is typically administered to women with minimal residual disease.
During IV administration, several drugs known as vesicants require special care (Table 27-1). Extravasation of these into the subcutaneous tissue can result in severe pain and necrosis. These drugs require slow infusion either through a rapidly flowing peripheral IV catheter or preferably via a central venous catheter. If extravasation is suspected, the infusion is immediately stopped, the affected arm elevated, and ice packs applied. In severe cases, a plastic surgeon should be consulted.
Agent activity and toxicity is influenced dramatically by drug inactivation, elimination, or excretion. For the most part, this takes place primarily via the liver or kidneys. As a result, drug activity may be diminished and toxicity exacerbated when normal hepatic or renal function is impaired. In addition, drug toxicity is often more pronounced in the elderly or malnourished. For example, a low serum creatinine level in cachectic women may not accurately reflect underlying renal function. If a carboplatin dose is calculated using this falsely low value, the amount may be excessive and result in considerable morbidity. Instead, a preset creatinine level may need to be selected (0.8 or 1.0 mg/dL) to aid safer dosing in some patients.
Most women who receive chemotherapy are prescribed medication for other noncancerous conditions, such as hypertension. Moreover, women also often receive analgesics, antiemetics, and antibiotics during chemotherapy. Most drug interactions are of little consequence, but some may lead to substantially altered drug toxicity. Drugs that are metabolized in the liver are particularly at risk for such interactions. For example, using methotrexate in a woman taking warfarin (Coumadin) will usually enhance the anticoagulant effect and thus will require a warfarin dose reduction.
An anaphylactic, allergic, or hypersensitivity reaction during or after administration of chemotherapy may develop, despite patient history review and administration of prophylactic medications. Accordingly, a treatment facility must have trained nursing staff and resources to manage these sudden, but common, issues. Prior to drug administration, a woman is instructed to report symptoms that may herald an anaphylactic reaction such as flushing, pruritus, dyspnea, tachycardia, hoarseness, or lightheadedness. Emergency equipment, such as supplemental oxygen, ventilatory face mask and bag, or intubation equipment, must be immediately available. For a localized hypersensitivity response, administration of intravenous diphenhydramine (Benadryl) and/or corticosteroids may be sufficient. However, for a generalized hypersensitivity or anaphylactic response, chemotherapy should be stopped immediately, the emergency team notified, and emergency drugs administered, such as epinephrine (0.1–0.5 mg of a 1:10,000 solution) (Table 27-2).
|
In principle, larger tumor masses have a greater proportion of cells that have already developed resistance. Resistance may be intrinsic or acquired, and it may develop to one drug or to multiple agents. Intrinsic drug resistance is seen if tumors are first exposed to an agent and fail to respond. In contrast, with acquired drug resistance, tumors no longer respond to drugs to which they were initially sensitive. Sometimes, this develops with a specific drug. More often, however, acquired resistance is “pleiotropic,” meaning that a cancer is resistant to multiple chemotherapy agents. This is often mediated by the P-glycoprotein or multidrug resistance pump. Advanced ovarian cancer is a good example. Most patients will initially achieve remission with platinum-based chemotherapy, but 80 percent will ultimately relapse and die from tumors that have become resistant to all cytotoxic therapy.
The effective use of chemotherapy is a dynamic process whereby a treating clinician is constantly weighing toxicity to the patient against tumor response. Numerous factors influence toxicity and include the patient’s baseline nutrition, overall health, extent of disease, and prior therapy. In counseling women to continue treatment or switch to a different regimen, a clinician must have objective criteria for response (Table 27-3). The most important indicator is the complete response rate. For ovarian cancer, this would include normal CA125 levels (usually <35 U/mL), physical examination findings, and imaging test results. Ultimately, women who have any possibility of cure are those who first achieve a complete response. However, if chemotherapy results in a partial response, many women still view this as advantageous compared with supportive care, even if a survival benefit is unproven.
| End Point | Definition |
|
|
CHEMOTHERAPEUTIC DRUGS
The antimetabolites are analogues of naturally occurring components of the metabolic pathways that lead to the synthesis of purines, pyrimidines, and nucleic acids. In most cases, they are S phase-specific agents that are most effective in rapidly growing tumors associated with short doubling times and large growth fractions (Table 27-4).
| Generic Name | Brand Name | Indications | Routes | Common Dosages | Common Toxicity |
| Methotrexate | Trexall, Rheumatrex | GTN | PO, IM, IV, intrathecal | IM: 1 mg/kg on days 1, 3, 5, 7 of 8-day cycle or 30–50 mg/m 2/wk IV: 100 mg/m 2 during 30 min, then 200 mg/m 2 during 12 hr | BMD, mucositis, renal toxicity, CNS dysfunction |
| Gemcitabine | Gemzar | Recurrent ovarian CA, uterine sarcoma | IV | 600–1250 mg/m 2/wk over 30 min × 2–3 wk | BMD, N/V/D, malaise and fever |
| 5-Fluorouracil | Adrucil Efudex | Cervical CA, vulvar CA VAIN | IV Vaginal cream | 800–1000 mg/m 2/d during 96 hr 3 mL QOD × 1 wk, then weekly up to 10 wk | Mucositis, PPE Vulvovaginal irritation |
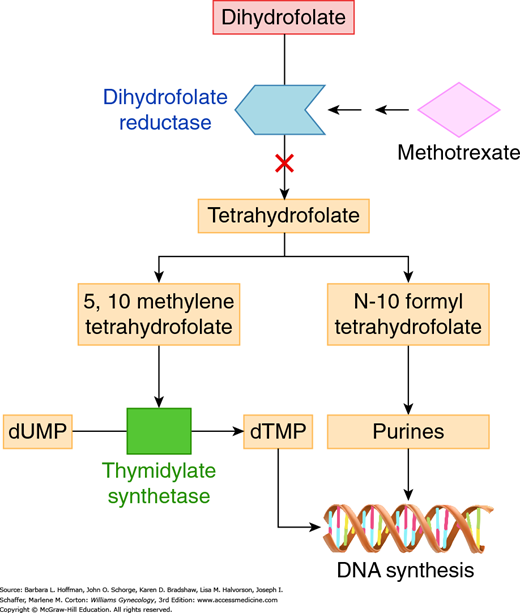
This antimetabolite is U.S. Food and Drug Administration (FDA)-approved to be used solely for treatment of women with gestational trophoblastic neoplasia (GTN). It is also commonly used for the medical management of ectopic pregnancy. Methotrexate (MTX) tightly binds to dihydrofolate reductase, blocking the reduction of dihydrofolate to tetrahydrofolate (the active form of folic acid) (Fig. 27-3). As a result, thymidylate synthetase and various steps in de novo purine synthesis are halted. This leads to arrest of DNA, RNA, and protein synthesis.
FIGURE 27-3
Methotrexate’s primary target is the enzyme dihydrofolate reductase (DHFR). Inhibition of DHFR leads to partial depletion of 5,10 methylene tetrahydrofolic acid and N-10 formyl tetrahydrofolic acid, which are cofactors required for the respective synthesis of thymidylate and purines. As a result, methotrexate leads to arrested DNA, RNA, and protein synthesis. dTMP = deoxythymidine monophosphate; dUMP = deoxyuridine monophosphate.
Methotrexate may be administered orally, IM, IV, or intrathecally. Most commonly, single-agent treatment of GTN involves MTX given IM as an 8-day regimen of 1 mg/kg on treatment days 1, 3, 5, and 7, or at dosages of 30 to 50 mg/m2 once each week. Combination therapy for high-risk disease includes 100 mg/m2 MTX given IV over 30 minutes, followed by a 200 mg/m2 IV dose over 12 hours. With MTX, patients are counseled to avoid folate-containing supplements unless specifically directed.
MTX causes few side effects at typical doses. However, at high doses, although used infrequently, this agent can lead to fatal bone marrow toxicity. This toxicity can be prevented by “rescue” doses of leucovorin. Leucovorin is folinic acid, has activity that is equivalent to folic acid, and thus is readily converted to tetrahydrofolate. Leucovorin, however, does not require dihydrofolate reductase for its conversion. Therefore, its function is unaffected by inhibition of this enzyme by MTX. Leucovorin administration, therefore, allows for some purine and pyrimidine synthesis. Leucovorin rescue is incorporated into the 8-day alternating MTX schedule, and a 0.1 mg/kg leucovorin dose is provided orally on treatment days 2, 4, 6, and 8.
In addition to myelosuppression, renal toxicity and acute cerebral dysfunction are typically only seen at high MTX doses. Methotrexate is predominantly excreted through the kidneys, and thus women with renal insufficiency have doses reduced. Serum MTX levels are carefully monitored in these patients, as they may require prolonged leucovorin rescue.
This antimetabolite is FDA approved to be used with other agents for treatment of recurrent ovarian cancer but is also commonly used for uterine sarcoma. Gemcitabine (Gemzar) is a synthetic nucleoside analogue that undergoes multiple phosphorylations to form the active metabolite. The resulting triphosphate is subsequently incorporated into DNA as a fraudulent base pair. Following the insertion of gemcitabine, one additional deoxynucleotide is added to the end of the DNA chain before replication is terminated, and thereby, DNA synthesis is halted.
The usual administration of gemcitabine is by 30-minute infusion. Longer durations, such as those greater than 60 min, are associated with increased toxicity due to intracellular accumulation of the triphosphate. Depending on whether it is used as a single agent or in combination, gemcitabine is typically given at doses between 600 and 1250 mg/m2 once weekly for 2 to 3 weeks, followed by a week off therapy.
Myelosuppression, especially neutropenia, is the main dose-limiting side effect. Gastrointestinal (GI) toxicity, such as nausea, vomiting, diarrhea, or mucositis, is also common. Approximately 20 percent of patients will develop a flulike syndrome, including fever, malaise, headache, and chills. Pulmonary toxicity is infrequent, but reported.
5-Fluorouracil (5-FU) is not FDA approved for gynecologic cancer but is occasionally paired with cisplatin during chemoradiation for cervical cancer. A topical form (Efudex) can be used for vaginal intraepithelial neoplasia (VAIN) treatment as discussed in Chapter 29. This “false” pyrimidine antimetabolite acts principally as a thymidine synthetase inhibitor to block DNA replication.
Systemic 5-FU (Adrucil) is usually given as a 96-hour continuous IV infusion of 800 to 1000 mg/m2/d. Mucositis and/or diarrhea may be severe and dose-limiting. Hand-foot syndrome (palmar-plantar erythrodysesthesia), described on page 600, is less common but can also be dose-limiting. Myelosuppression, mainly neutropenia and thrombocytopenia, are less frequent. Nausea and vomiting are usually mild.
The class of alkylating agents is characterized by positively charged alkyl groups that bind to negatively charged DNA to form adducts (Table 27-5). Binding leads to DNA breaks or cross-links and a halt to DNA synthesis. In general, these drugs are cell cycle-nonspecific agents.
| Generic Name | Brand Name | Indication | Routes | Dosages | Toxicity |
| Cyclophosphamide | Cytoxan | GTN, recurrent ovarian CA | PO, IV | IV: 500–750 mg/m 2 over 30 min, every 3 wk PO: 50 mg/d | BMD, cystitis, N/V, alopecia |
| Ifosfamide | Ifex | Recurrent ovarian CA, cervical CA, uterine sarcoma | IV | 1.2–1.6 g/m 2/d, days 1–3 of 3-wk cycle | BMD, cystitis, N/V, alopecia, CNS and renal toxicity |
Of alkylating agents, cyclophosphamide (Cytoxan) is FDA approved by itself or in combination for epithelial ovarian cancer treatment. Cyclophosphamide is the “C” of the EMA-CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, Oncovin [vincristine]), which is a regimen prescribed for high-risk GTN. It is also used, albeit infrequently, as salvage therapy for recurrent epithelial ovarian cancer (Bower, 1997; Cantu, 2002). Cyclophosphamide is a derivative of nitrogen mustard and is activated through a multistep process by microsomal enzymes in the liver. It promotes DNA cross-linking and DNA synthesis inhibition.
This agent may be administered IV or orally. It is typically given IV at doses of 500 to 750 mg/m2 over 30 minutes every 3 weeks. Orally, a metronomic (repetitive low-dose) regimen of 50 mg daily is often used to minimize toxicity and target the tumor endothelium or stroma in combination with a biologic agent, such as bevacizumab (Chura, 2007).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree