INTRODUCTION
Pelvic organ prolapse is a common condition that can lead to genital tract dysfunction and diminished quality of life. Signs include descent of one or more of the following: the anterior vaginal wall, posterior vaginal wall, uterus and cervix, vaginal apex, or the perineum (Haylen, 2010). Symptoms include vaginal bulging, pelvic pressure, and splinting or digitation. Splinting is manual bolstering of the prolapse to improve symptoms, whereas digitation aids stool evacuation. For pelvic organ prolapse to be considered a disease state in a given individual, symptoms should be attributable to pelvic organ descent such that surgical or nonsurgical reduction relieves the symptoms, restores function, and improves quality of life.
EPIDEMIOLOGY
Pelvic organ prolapse (POP) affects millions of women worldwide. In the United States, it is the third most common indication for hysterectomy. Moreover, a woman has an estimated cumulative lifetime risk of 12 percent to undergo surgery for POP (Wu, 2014). Estimates of disease prevalence are hampered by lack of consistent definitions. If the validated Pelvic Organ Prolapse Quantification examination alone is used to describe pelvic organ support, 30 to 65 percent of women presenting for routine gynecologic care have stage 2 prolapse (Bland, 1999; Swift, 2000, 2005; Trowbridge, 2008). In contrast, studies that define prolapse solely based on patient symptoms show a prevalence ranging from 3 to 6 percent in the United States (Bradley, 2005; Nygaard, 2008; Rortveit, 2007).
RISK FACTORS
Table 24-1 summarizes predisposing factors for POP. It develops gradually over a span of years, and its etiology is multifactorial. The relative importance, however, of each factor is not known.
| Pregnancy |
| Vaginal childbirth |
| Menopause |
| Aging |
| Hypoestrogenism |
| Chronically increased intraabdominal pressure |
| Chronic obstructive pulmonary disease |
| Constipation |
| Obesity |
| Pelvic floor trauma |
| Genetic factors |
| Race |
| Connective tissue disorders |
| Spina bifida |
Of these, vaginal childbirth is the most frequently cited risk factor. Some evidence suggests that pregnancy itself predisposes to POP. But numerous studies have clearly shown that vaginal delivery increases a woman’s propensity for developing POP. In the Pelvic Organ Support Study (POSST), increasing parity was associated with prolapse risk (Swift, 2005). Specifically, the risk of POP increased 1.2 times with each vaginal delivery. In the Reproductive Risks for Incontinence Study at Kaiser (RRISK) study, Rortveit and colleagues (2007) found that the prolapse risk increased significantly in woman with one vaginal delivery (odds ratio [OR] 2.8), two (OR 4.1), or three or more (OR 5.3) deliveries compared with nulliparas. In a longitudinal study of 1011 women, vaginal delivery was associated with a significantly greater risk of prolapse to the hymen or beyond compared with cesarean delivery without labor (OR 5.6) (Handa, 2011).
Although vaginal delivery is implicated in a woman’s lifetime risk for POP, specific obstetric risk factors remain controversial. These include macrosomia, prolonged second-stage labor, episiotomy, anal sphincter laceration, epidural analgesia, forceps use, and oxytocin stimulation of labor. Each is a proposed risk factor. As we await further studies, we can anticipate that although each may have an important effect, it is the cumulative sum of all events occurring as the fetus traverses the birth canal that predisposes to POP.
Currently, two obstetric interventions—elective forceps delivery to shorten second-stage labor and elective episiotomy—are not advocated. Both lack evidence of benefit and carry risks for maternal and fetal harm. First, forceps delivery is directly implicated in pelvic floor injury through its association with anal sphincter laceration. Additionally, recent evidence shows that operative vaginal birth significantly increases the odds for all pelvic floor disorders, especially prolapse (OR 7.5) (Handa, 2011). For these reasons, elective forceps delivery is not recommended to prevent pelvic floor disorders and may be a contributing factor. Likewise, at least six randomized controlled trials (RCTs) comparing elective and selective episiotomy have shown no proven benefit. These studies have shown an association with anal sphincter laceration, postpartum anal incontinence, and postpartum pain (Carroli, 2009).
Elective cesarean delivery to prevent pelvic floor disorders such as POP and urinary incontinence is controversial. Theoretically, if all women underwent cesarean delivery, fewer women would have pelvic floor disorders. Keeping in mind that most women do not have these disorders, cesarean delivery on maternal request (CDMR) would subject many women to a potentially dangerous intervention who would otherwise not develop the problem. Specifically, given the 12-percent lifetime risk of undergoing surgery for prolapse, for every one woman who would avoid pelvic floor surgery later in life by undergoing primary elective cesarean delivery, approximately nine women would gain no benefit yet would nevertheless assume the potential risks of cesarean delivery. Definitive recommendations will require further studies to define the potential risks and benefits of CDMR for primary prevention of pelvic floor dysfunction (American College of Obstetricians and Gynecologists, 2013b; Patel, 2006). Currently, decisions regarding CDMR to prevent pelvic floor disorders must be individualized. That said, the American College of Obstetricians and Gynecologists (2013a) recommends against CMDR for women desiring several children given the risk of abnormal placentation with accruing cesarean deliveries.
Data from several studies show that POP prevalence increases steadily with age (Nygaard, 2008; Olsen, 1997; Swift, 2005). In the POSST study, in women aged 20 to 59 years, the incidence of POP roughly doubled with each decade. As with other risks for POP, aging is a complex process. The increased incidence may result from physiologic aging and degenerative processes and from hypoestrogenism. Research clearly demonstrates an important role for reproductive hormones in the maintenance of connective tissues and the extracellular matrix necessary for pelvic organ support. Estrogen and progesterone receptors have been identified in the nuclei of connective tissue and smooth muscle cells of both the levator ani stroma and uterosacral ligaments (Smith, 1990, 1993). Separating the effects of estrogen deprivation from the effects of the aging process is problematic.
Women with connective tissue disorders may be more likely to develop POP. Histologic studies have shown that in women with POP, the ratio of collagen I to collagen III and IV is decreased (Moalli, 2004). This relative decline in well-organized dense collagen is believed to contribute to weakening of vaginal wall tensile strength and an increased susceptibility to vaginal wall prolapse. In a small case series study, one third of women with Marfan syndrome and three fourths of women with Ehlers-Danlos syndrome reported a history of POP (Carley, 2000).
Racial differences in POP prevalence have been demonstrated in several studies (Schaffer, 2005). Black and Asian women show the lowest risk, whereas Hispanic and white women appear to have the highest risk (Hendrix, 2002; Kim, 2005; Whitcomb, 2009). Although differences in collagen content have been demonstrated between races, racial differences in the bony pelvis may also play a role. For instance, black women more commonly have a narrow pubic arch and an android or anthropoid pelvis. These shapes are protective against POP compared with the gynecoid pelvis typical of most white women.
In addition, emerging evidence suggests that POP may have a genetic component. Recent genome-wide linkage studies have identified specific predisposition genes that may contribute to POP (Allen-Brady, 2015).
Chronically elevated intraabdominal pressure is believed to play a role in POP pathogenesis. Elevated pressures are present with obesity, chronic constipation, chronic coughing, and repetitive heavy lifting. Higher body mass index (BMI) correlates with POP risk. In the Women’s Health Initiative (WHI) trial, being overweight (BMI 25 to 30 kg/m2) increased the POP rate by 31 to 39 percent, and obesity (BMI >30 kg/m2) raised the POP rate 40 to 75 percent (Hendrix, 2002). With regard to lifting, a Danish study demonstrated that nursing assistants who were involved with repetitive heavy lifting were at increased risk to undergo surgical intervention for prolapse (OR 1.6) (Jorgensen, 1994). In addition, cigarette smoking and chronic obstructive pulmonary disease (COPD) have also been implicated in POP development (Gilpin, 1989; Olsen, 1997). In a matched case-control study, COPD was associated with an increased risk of future pelvic floor repair after hysterectomy (Blandon, 2009). The repetitive increases in intraabdominal pressure resulting from chronic coughing may predispose to POP. Instead, some believe that the inhaled chemical compounds in tobacco may cause tissue changes that lead to POP rather than the chronic cough itself (Wieslander, 2005).
DESCRIPTION AND CLASSIFICATION
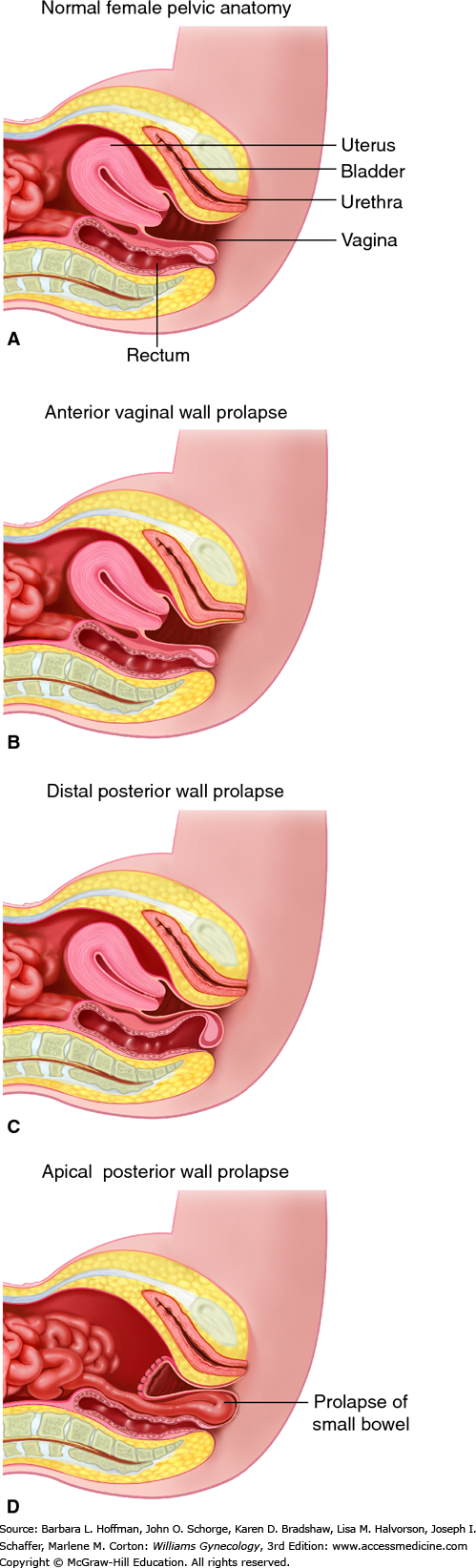
Pelvic organ prolapse is descent of the anterior vaginal wall, posterior vaginal wall, uterus (cervix), the vaginal apex after hysterectomy, rectum, or the perineum, alone or in combination. The terms cystocele, cystourethrocele, uterine prolapse, uterine procidentia, rectocele, and enterocele have traditionally been used to describe the structures behind the vaginal wall thought to be prolapsed (Fig. 24-1). However, these terms are imprecise and misleading, as they focus on what is presumed to be prolapsed rather than what is objectively noted to be prolapsed.
Although these terms are deeply entrenched in the literature, it is more clinically useful to describe prolapse in terms of what one actually sees: anterior vaginal wall prolapse, apical prolapse, cervical prolapse, posterior vaginal wall prolapse, rectal prolapse, or perineal descent.
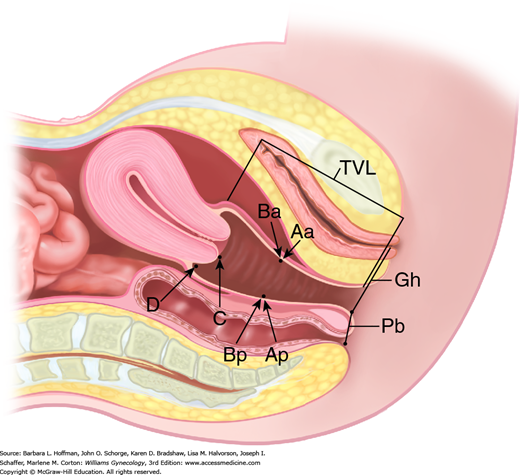
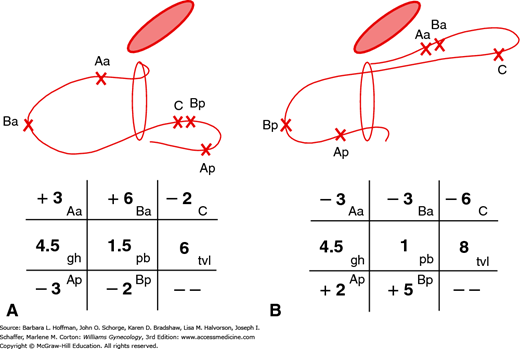
In 1996, the International Continence Society defined a system of Pelvic Organ Prolapse Quantification (POP-Q) (Bump, 1996). Demonstrating high intra- and interexaminer reliability, the POP-Q system allows clinicians and researchers to report findings in a standardized, easily reproducible fashion. This system contains a series of site-specific measurements of a woman’s pelvic organ support. Prolapse in each segment is measured relative to the hymen, which is an anatomic landmark that can be identified consistently. Six points are located with reference to the plane of the hymen: two on the anterior vaginal wall (points Aa and Ba), two at the apical vagina (points C and D), and two on the posterior vaginal wall (points Ap and Bp) (Fig. 24-2). The genital hiatus (Gh), perineal body (Pb), and total vaginal length (TVL) are also measured. All POP-Q points, except TVL, are measured during patient Valsalva and should reflect maximum protrusion.
Point Aa defines a point that lies in the midline of the anterior vaginal wall and is 3 cm proximal to the external urethral meatus. This corresponds to the proximal location of the urethrovesical crease. In relation to the hymen, this point’s position ranges from –3 (normal support) to +3 cm (maximum prolapse of point Aa).
Point Ba represents the most distal position of any part of the upper anterior vaginal wall, that is, the segment of vagina that normally would extend cephalad from point Aa. It is –3 cm in the absence of prolapse. In a woman with total vaginal eversion posthysterectomy, Ba would have a positive value equal to the position of the cuff from the hymen.
The two apical points, C and D, which are located in the proximal vagina, represent the most cephalad locations of a normally positioned lower reproductive tract. Point C defines a point that is at either the most distal edge of the cervix or the leading edge of the vaginal cuff after total hysterectomy.
Point D defines a point that represents the location of the posterior fornix in a woman who still has a cervix. It is omitted in the absence of a cervix. This point represents the level of uterosacral ligament attachment to the proximal posterior cervix and thus differentiates uterosacral-cardinal ligament support failure from cervical elongation. The total vaginal length (TVL) is the greatest depth of the vagina in centimeters when point C or D is reduced to its fullest position.
Point Ap defines a point in the midline of the posterior vaginal wall that lies 3 cm proximal to the hymen. Relative to the hymen, this point’s range of position is by definition –3 (normal support) to +3 cm (maximum prolapse of point Ap).
Point Bp represents the most distal position of any part of the upper posterior vaginal wall. By definition, this point is at –3 cm in the absence of prolapse. In a woman with total vaginal eversion posthysterectomy, Bp would have a positive value equal to the position of the cuff from the hymen.
In addition to the hymen, remaining measurements include those of the genital hiatus (Gh) and the perineal body (Pb) (see Fig. 24-2). The genital hiatus is measured from the middle of the external urethral meatus to the midline of the posterior hymenal ring. The perineal body is measured from the posterior margin of the genital hiatus to the midanal opening.
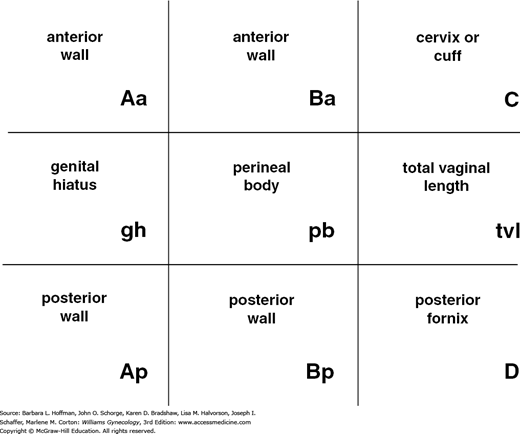
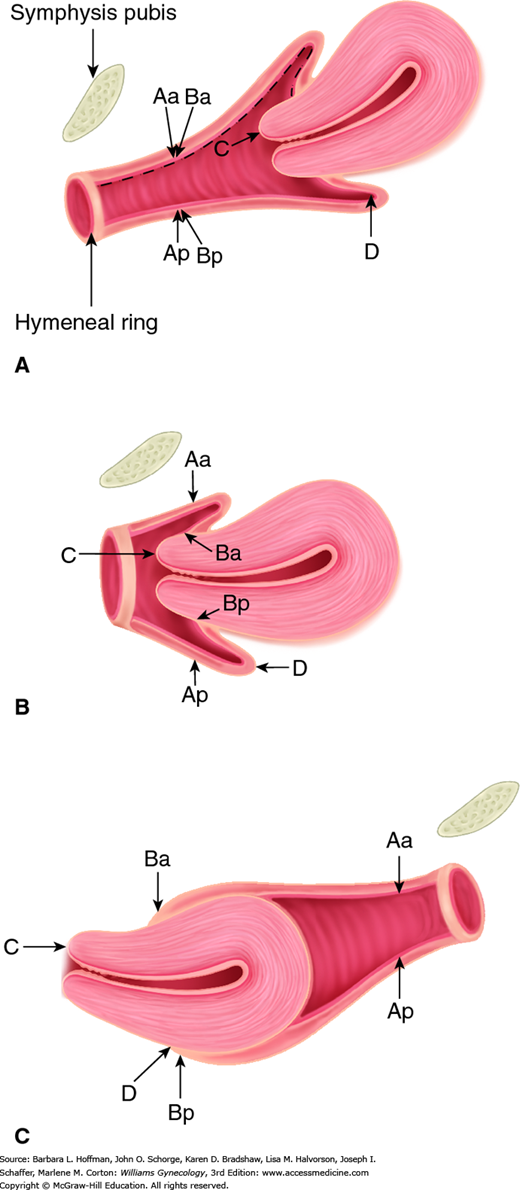
With the hymenal plane defined as zero, the anatomic position of these points from the hymen is measured in centimeters. Points above or proximal to the hymen are described with a negative number. Positions below or distal to the hymen are noted using a positive number. The point measurements can be organized using a three-by-three grid as shown in Figure 24-3. Figures 24-4 and 24-5 illustrate the use of POP-Q in evaluating different examples of POP.
FIGURE 24-5
Grid and drawing of an anterior support defect (A) and posterior support defect (B). (Reproduced with permission from Bump RC, Mattiasson A, Bø K, et al: The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction, Am J Obstet Gynecol 1996 Jul;175(1):10–17.)
The degree of prolapse can also be quantified using a five-stage ordinal system as summarized in Table 24-2 (Bump, 1996). Stages are assigned according to the most severe portion of the prolapse.
| Stage 0: | No prolapse is demonstrated. Points Aa, Ap, Ba, and Bp are all at −3 cm and either point C or D is between −TVL (total vaginal length) cm and −(TVL −2) cm (i.e., the quantitation value for point C or D is ≤−[TVL −2] cm). Figure 24-2 represents stage 0 |
| Stage I: | The criteria for stage 0 are not met, but the most distal portion of the prolapse is >1 cm above the level of the hymen (i.e., its quantitation value is <−1 cm) |
| Stage II: | The most distal portion of the prolapse is ≤1 cm proximal to or distal to the plane of the hymen (i.e., its quantitation value is ≥−1 cm but ≤+1 cm) |
| Stage III: | The most distal portion of the prolapse is >1 cm below the plane of the hymen but protrudes no further than 2 cm less than the total vaginal length in centimeters (i.e., its quantitation value is >+1 cm but <+[TVL −2] cm). Figure 24-5B represents stage III Bp prolapse |
| Stage IV: | Essentially, complete eversion of the total length of the lower genital tract is demonstrated. The distal portion of the prolapse protrudes to at least (TVL − 2) cm (i.e., its quantitation value is ≥+[TVL − 2] cm). In most instances, the leading edge of stage IV prolapse will be the cervix or vaginal cuff scar. Figure 24-4C represents stage IVC prolapse |
This descriptive tool is also used to classify prolapse during physical examination and is widely used. Although not as informative as the POP-Q, it is adequate for clinical use if each compartment (anterior, apical, and posterior) is evaluated (Table 24-3) (Baden, 1972).
| Grade | |
| Grade 0 | Normal position for each respective site |
| Grade 1 | Descent halfway to the hymen |
| Grade 2 | Descent to the hymen |
| Grade 3 | Descent halfway past the hymen |
| Grade 4 | Maximum possible descent for each site |
PATHOPHYSIOLOGY
Pelvic organ support is maintained by complex interactions among the pelvic floor muscles, pelvic floor connective tissue, and vaginal wall. These work in concert to provide support and also maintain normal physiologic function of the vagina, urethra, bladder, and rectum. Several factors are implicated in failure of this support, but none fully explain its pathogenesis. These include genetic predisposition, loss of pelvic floor striated muscle support, vaginal wall weakness, and loss of connective attachments between the vaginal wall and the pelvic floor muscles and pelvic viscera. As noted earlier, vaginal birth and aging are two major risk factors for POP (Mant, 1997). The loss of support that evolves decades after vaginal delivery may stem from an initial insult compounded by aging and other contributors.
The levator ani muscle is a pair of striated muscles composed of three regions. The iliococcygeus muscle forms a flat horizontal shelf spanning from one pelvic sidewall to the other (Figs. 38-7 and 38-8). The pubococcygeus muscle arises from the pubic bone on either side; is attached to the walls of the vagina, urethra, anus, and perineal body; and inserts on the coccyx. The pubococcygeus muscle thereby helps suspend the vaginal wall to the pelvis. The puborectalis muscle forms a sling that originates from the pubic bone. This wraps around and behind the rectum. Connective tissue covers the superior and inferior fascia of the levator ani muscle. In the healthy state, baseline resting contractile activity of the levator ani muscle elevates the pelvic floor and compresses the vagina, urethra, and rectum toward the pubic bone (Fig. 38-10). This narrows the genital hiatus and prevents prolapse of the pelvic organs.
When the levator ani muscle has normal tone and the vagina has adequate depth, the upper vagina lies nearly horizontal in the standing female. Thus, during periods of increased intraabdominal pressure, the upper vagina is compressed against the levator plate. It is theorized that when the levator ani muscle loses tone, the vagina drops from a horizontal to a semivertical position (Fig. 38-11). This widens or opens the genital hiatus and predisposes pelvic viscera to prolapse. Without adequate levator ani muscle support, the visceral fascial attachments of the pelvic contents are placed on tension and are thought to stretch and eventually fail.
Theoretically, the levator ani muscle may sustain either direct muscle or denervation injury during childbirth, and these injuries are involved in POP pathogenesis. During second-stage labor, nerve injury from stretch or compression or both is believed to partially denervate the levator ani muscle. Denervated muscle loses tone, the genital hiatus opens, and pelvic viscera prolapse (DeLancey, 1993; Peschers, 1997; Shafik, 2000).
The experimental evidence for this theory of denervation-induced injury leading to POP has been difficult to obtain and is contradictory. Some studies demonstrate histomorphologic abnormalities in the levator ani muscle from women with prolapse and stress incontinence, whereas other studies fail to find histologic evidence of levator ani muscle denervation (Gilpin, 1989; Hanzal, 1993; Heit, 1996; Koelbl, 1989). In addition, levator ani muscle biopsies obtained from parous and nulliparous cadavers failed to find evidence of atrophy or other important muscle changes (Boreham, 2009). This suggests that pregnancy and parturition have little or no effect on levator ani muscle histomorphology. Additionally, experimental denervation of the levator ani muscle in the squirrel monkey led to significant muscular atrophy but did not affect pelvic organ support. Taken together, experimental evidence does not support a role for denervation-induced injury in POP pathophysiology.
Importantly, however, loss of skeletal muscle volume and function occurs in virtually all striated muscles during aging. Results obtained from young and older women with POP indicate that the levator ani muscle undergoes substantial morphologic and biochemical changes during aging. Thus, loss of levator tone with age may contribute to pelvic organ support failure in older women, possibly those with preexisting defects in connective tissue support. As striated muscles lose tone, ligamentous and connective tissue support of the pelvic organs must sustain more forces conferred by abdominal pressure. As connective tissues bear these loads for long periods, they stretch and may eventually fail, resulting in prolapse.
A continuous interdependent system of connective tissues and ligaments surrounds the pelvic organs and attaches them to the levator ani muscle and bony pelvis. The connective tissue of the pelvis is comprised of collagen, elastin, smooth muscle, and microfibers, which are anchored in an extracellular matrix of polysaccharides. The connective tissue that invests the pelvic viscera provides substantial pelvic organ support.
Of these, the arcus tendineus fascia pelvis is a condensation of parietal fascia covering the medial aspects of the obturator internus and levator ani muscle (Fig. 38-7). It provides the lateral and apical anchor sites for the anterior and posterior vagina. The arcus tendineus fascia pelvis is therefore poised to withstand descent of the anterior vaginal wall, vaginal apex, and proximal urethra. Experts now believe that a major inciting factor for prolapse is loss of connective tissue support at the vaginal apex leading to stretching or tearing of the arcus tendineus fascia pelvis. The result is apical and anterior vaginal wall prolapse.
The uterosacral ligaments contribute to apical support by suspending and stabilizing the uterus, cervix, and upper vagina. The ligament contains approximately 20 percent smooth muscle. Several studies have shown a decrease in the fractional area and distribution of smooth muscle in the uterosacral ligaments of women with prolapse (Reisenauer, 2008; Takacs, 2009). These studies suggest that abnormalities in uterosacral ligament support of the pelvic organs contribute to prolapse development.
Abnormalities of connective tissue and connective tissue repair may predispose women to prolapse (Norton, 1995; Smith, 1989). As noted, women with connective tissue disorders such as Ehlers-Danlos or Marfan syndrome are more likely to develop POP and urinary incontinence (Carley, 2000; Norton, 1995).
The pelvic floor fascia and connective tissues may also lose strength consequent to aging and loss of neuroendocrine signaling in pelvic tissues (Smith, 1989). Estrogen deficiency can affect the biomedical composition, quality, and quantity of collagen. Estrogen influences collagen content by increasing synthesis or decreasing degradation. Exogenous estrogen supplementation has been found to increase the skin collagen content in postmenopausal women who are estrogen deficient (Brincat, 1983). Moreover, estrogen supplementation prior to prolapse surgery and/or postoperatively is considered essential by many pelvic reconstruction surgeons. In a systematic review, Rahn and coworkers (2015) found that vaginal estrogen application before POP surgery improved the vaginal maturation index and increased vaginal epithelial thickness. This suggests a possible role in healing and future support. Although this practice may seem logical and empirically sound, evidence does not yet show improved surgical outcomes with this use of adjuvant estrogen.
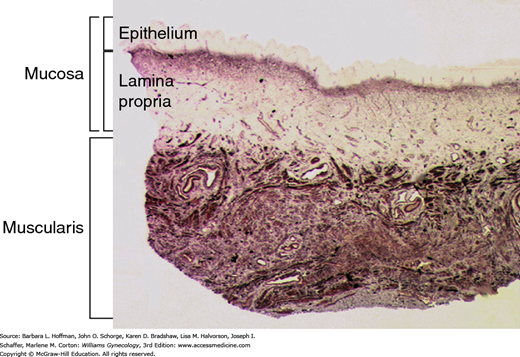
Abnormalities in the vaginal wall and its attachments to the pelvic floor muscles may be involved in POP pathogenesis. The vaginal wall is composed of mucosa (epithelium and lamina propria), a fibroelastic muscularis layer, and an adventitial layer that is made up of loose areolar tissue, abundant elastic fibers, and neurovascular bundles (Fig. 24-6). The muscularis and adventitial layers together form the fibromuscular layer, which was previously referred to as “endopelvic fascia.” The fibromuscular layer coalesces laterally and attaches to the arcus tendineus fascia pelvis and superior fascia of the levator ani muscle. In the lower third of the vagina, the vaginal wall is attached directly to the perineal membrane and the perineal body. This suspensory system, together with the uterosacral ligaments, prevents the vagina and uterus from descent when the genital hiatus is open.
FIGURE 24-6
Photomicrograph shows a cross section of the vaginal wall. Mucosal and muscularis layers are shown here. The adventitia, which is typically seen deep to muscularis, is not shown in this section. The fibromuscular layer is comprised of muscularis and adventitial layers. (Used with permission from Dr. Ann Word.)
Abnormalities in the anatomy, physiology, and cellular biology of vaginal wall smooth muscle may contribute to POP. Specifically, in fibromuscular tissue taken at the vaginal apex from both the anterior and posterior vaginal walls, vaginal prolapse is associated with loss of smooth muscle, myofibroblast activation, abnormal smooth muscle phenotype, and increased protease activity (Boreham, 2001, 2002a,b; Moalli, 2005; Phillips, 2006). Additionally, abnormal synthesis or degradation of vaginal wall collagen and elastin fibers appears to contribute to prolapse.
This theory states that tears in different sites of the “endopelvic fascia” surrounding the vaginal wall allow herniation of the pelvic organs. Specifically, attenuation of the vaginal wall without loss of fascial attachments is called a distention cystocele or rectocele (Fig. 24-7). With distention-type prolapse, the vaginal wall appears smooth and without rugae, due to abdominal contents pressed against the vagina from within. In contrast, anterior and posterior wall defects due to loss of the connective tissue attachment of the lateral vaginal wall to the pelvic sidewall are described as displacement (paravaginal) cystocele or rectocele (Fig. 24-8). With displacement-type prolapse, vaginal rugae are visible. Both defect types could result from the stretching or tearing of support tissues during second-stage labor.
Many experts now believe the primary defect leading to prolapse is loss of support at the vaginal apex. This allows the apical portions of the anterior and posterior vaginal walls to descend. As such, resuspension of the vaginal apex will restore support to both the anterior and posterior walls.
The vagina is a fibromuscular, flattened, cylindrical tube with three levels of support, as described by DeLancey (1992). Level I support suspends the upper or proximal vagina. Level II support attaches the midvagina along its length to the arcus tendineus fascia pelvis. Level III support results from fusion of the distal vagina to adjacent structures. Defects in each level of support result in identifiable vaginal wall prolapse: apical, anterior, and posterior.
Level I support consists of the cardinal and uterosacral ligaments attachment to the cervix and upper vagina (Fig. 38-15). The cardinal ligaments fan out laterally and attach to the parietal fascia of the obturator internus and piriformis muscles, the anterior border of the greater sciatic foramen, and the ischial spines. The uterosacral ligaments are posterior fibers that attach to the presacral region at the level of S2 through S4. Together, this dense visceral connective tissue complex maintains vaginal length and horizontal axis. It allows the vagina to be supported by the levator plate and positions the cervix just superior to the level of the ischial spines. Defects in this support complex may lead to apical prolapse. This is frequently associated with small bowel herniation into the vaginal wall, that is, enterocele.
Level II support consists of the paravaginal attachments that are contiguous with the cardinal/uterosacral complex at the ischial spine. These are the connective tissue attachments of the lateral vagina anteriorly to the arcus tendineus fascia pelvis and posteriorly to the arcus tendineus rectovaginalis. Detachment of this connective tissue from the arcus tendineus fascia pelvis leads to lateral or paravaginal anterior vaginal wall prolapse.
Level III support is composed of the perineal body, superficial and deep perineal muscles, and fibromuscular connective tissue. Collectively, these support the distal one third of the vagina and introitus. The perineal body is essential for distal vaginal support and proper function of the anal canal. Damage to level III support contributes to anterior and posterior vaginal wall prolapse, gaping introitus, and perineal descent.
PATIENT EVALUATION
Pelvic organ prolapse involves multiple anatomic and functional systems and is commonly associated with genitourinary, gastrointestinal, and musculoskeletal symptoms (Table 24-4). Prolapse rarely creates severe morbidity or mortality, however, it can greatly diminish quality of life. In contrast, many women with mild to advanced prolapse lack bothersome symptoms. Thus, initial evaluation must include a careful assessment of prolapse-related symptoms and their effect on activities of daily living.
| Symptoms | Other Possible Causes |
| Bulge symptoms | |
| Sensation of vaginal bulging or protrusion | Rectal prolapse |
| Seeing or feeling a vaginal or perineal bulge | Vulvar or vaginal cyst/mass |
| Pelvic or vaginal pressure | Pelvic mass |
| Heaviness in pelvis or vagina | Hernia (inguinal or femoral) |
| Urinary symptoms | |
| Urinary incontinence | Urethral sphincter incompetence |
| Urinary frequency | Detrusor overactivity |
| Urinary urgency | Hypoactive detrusor function |
| Weak or prolonged urinary stream | Bladder outlet obstruction (i.e., postsurgical) |
| Hesitancy | Excessive fluid intake |
| Feeling of incomplete emptying | Interstitial cystitis |
| Manual reduction of prolapse to start or complete voiding | Urinary tract infection |
| Position change to start or complete voiding | |
| Bowel symptoms | |
| Incontinence of flatus or liquid/solid stool | Anal sphincter disruption or neuropathy |
| Feeling of incomplete emptying | Diarrheal disorder |
| Hard straining to defecate | Rectal prolapse |
| Urgency to defecate | Irritable bowel syndrome |
| Digital evacuation to complete defecation | Rectal inertia |
| Splinting vagina or perineum to start or complete defecation | Pelvic floor dyssynergia |
| Feeling of blockage or obstruction during defecation | Hemorrhoids |
| Anorectal neoplasm | |
| Sexual symptoms | |
| Dyspareunia | Vaginal atrophy |
| Decreased lubrication | Levator ani syndrome |
| Decreased sensation | Vulvodynia |
| Decreased arousal or orgasm | Other female sexual disorder |
| Pain | |
| Pain in vagina, bladder, or rectum | Interstitial cystitis |
| Pelvic pain | Levator ani syndrome |
| Low back pain | Vulvodynia |
| Lumbar disc disease | |
| Musculoskeletal pain | |
| Other causes of chronic pelvic pain | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree