DEFINITIONS
Urinary incontinence is defined as involuntary leakage of urine. This is in contrast to urine that leaks from extraurethral sources, such as with fistulas or congenital malformations of the lower urinary tract. Although incontinence is categorized into several forms, this chapter focuses on the evaluation and management of stress and urgency urinary incontinence. Stress urinary incontinence (SUI) is the involuntary leakage of urine with increases in intraabdominal pressure. Urgency urinary incontinence (previously “urge” urinary incontinence) is the involuntary leakage accompanied or immediately preceded by a perceived strong imminent need to void. A related condition, overactive bladder, describes urinary urgency with or without incontinence and usually with increased daytime urinary frequency and nocturia (Abrams, 2009).
According to International Continence Society guidelines, urinary incontinence is a symptom, a sign, and a condition (Abrams, 2002). For example, with SUI, a patient may complain of involuntary urine leakage with exercise or laughing. Concurrent with these symptoms, involuntary leakage from the urethra synchronous with cough or Valsalva may be observed during examination by a provider. And as a condition, SUI is objectively demonstrated during urodynamic testing if involuntary leakage of urine is seen with increased abdominal pressure and absence of detrusor muscle contraction. Under these circumstances, when the symptom or sign of SUI is confirmed with objective testing, the term urodynamic stress incontinence is preferred.
With urgency urinary incontinence, women have difficulty postponing urination urges and generally must promptly empty their bladder on cue and without delay. Common triggers are hand washing, running water, or exposure to cold. Urgency urinary incontinence is sometimes objectively demonstrated during urodynamic testing to correspond temporally with spontaneous detrusor muscle contractions—a condition termed detrusor overactivity. When both stress and urgency symptoms are present, it is called mixed urinary incontinence.
EPIDEMIOLOGY
In Western societies, epidemiologic studies indicate a prevalence of urinary incontinence of 25 to 51 percent and even higher among nursing home patients (Buckley, 2010; Markland, 2011). This wide range is attributed to variations in research methodologies, population characteristics, and definitions of incontinence. As part of the 2005 to 2006 National Health and Nutrition Examination Survey (NHANES), a cross-sectional group of 1961 nonpregnant, noninstitutionalized women in the United States were questioned regarding pelvic floor disorders. Urinary incontinence characterized by participants as moderate to severe leakage was identified in 15.7 percent (Nygaard, 2008). However, current available data are limited by the fact that most women do not seek medical attention for this condition (Hunskaar, 2000). It is estimated that only one in four women will seek medical advice for incontinence, due to embarrassment, limited health care access, or poor screening by health care providers (Hagstad, 1985).
Among ambulatory women with urinary incontinence, the most common type is SUI, which represents 29 to 75 percent of cases. Urgency urinary incontinence accounts for up to 33 percent of incontinence cases, whereas the remainder is attributable to mixed forms (Hunskaar, 2000). In one review, 15 percent of 64,528 women met criteria for overactive bladder with or without incontinence, and 11 percent had urgency urinary incontinence (Hartmann, 2009).
Urinary incontinence can significantly impair quality of life and lead to disrupted social relationships, embarrassment and frustration, hospitalizations due to skin breakdown and urinary tract infection (UTI), and nursing home admission. An incontinent elderly woman is 2.5 times more likely to be admitted to a nursing home than a continent one (Langa, 2002). Moreover, population projections from the U.S. Census Bureau forecast that the number of American women with urinary incontinence will increase 55 percent from 18.3 million to 28.4 million between 2010 and 2050 (Wu, 2009).
RISKS
The prevalence of incontinence appears to increase gradually during young adult life. For example, data from the 2005 to 2006 NHANES demonstrate a steady increase in incontinence prevalence with age: 7 percent in those aged 20 to 40 years, 17 percent for ages 40 to 60, 23 percent for ages 60 to 80, and 32 percent for those older than 80 (Nygaard, 2008).
Incontinence should not be viewed as a normal consequence of aging. However, several physiologic age-related changes in the lower urinary tract may predispose to incontinence, overactive bladder, or other voiding difficulties. First, the prevalence of involuntary detrusor contractions increases with age, and detrusor overactivity is found in 21 percent of healthy, continent community-dwelling elderly (Resnick, 1995). Both total bladder capacity and the ability to postpone voiding decreases, and these declines may lead to urinary frequency. In addition, urinary flow rates are reduced in older women and are likely due to an age-associated decrease in detrusor contractility (Resnick, 1984). In women, postmenopausal decreases in estrogen levels result in atrophy of the urethral mucosal seal, loss of compliance, and bladder irritation, which may predispose to both stress and urgency urinary incontinence. Finally, renal filtration rate and diurnal levels of antidiuretic hormone and atrial natriuretic factor change with age. These alterations shift the diurnal-predominant pattern of fluid excretion toward one with greater urine excretion later in the day (Kirkland, 1983).
Race may influence incontinence rates, and white women are believed to have higher SUI rates than women of other races. In contrast, urgency urinary incontinence is believed to be more prevalent among African-American women. Most reports are not population-based and thus are not the best estimate of true racial differences. However, data from the Nurses’ Health Study cohorts, which included more than 76,000 women, did support these racial differences (Townsend, 2010). It is not yet clear whether these differences are biologic, related to health-care access, or affected by cultural expectations and symptom tolerance thresholds.
Body mass index (BMI) is a significant and independent risk factor for urinary incontinence of all types (Table 23-1). Specifically, the prevalence of both urgency urinary and stress incontinence increases proportionally with BMI (Hannestad, 2003). Theoretically, the increase in intraabdominal pressure that coincides with an increased BMI results in a higher intravesical pressure. This higher pressure overcomes urethral closing pressure and leads to incontinence (Bai, 2002). Encouragingly, weight loss for many can be an effective treatment and is considered a first-line option to reduce urinary incontinence rates (Dumoulin, 2014b). The prevalence of urinary incontinence significantly declines following weight loss achieved by behavior modification or with bariatric surgery (Burgio, 2007; Deitel, 1988; Subak, 2009). Even losses of 5 to 10 percent of body weight are sufficient for significant improvement in urinary incontinence (Wing, 2010).
Menopause may have a relationship with incontinence, but studies have inconsistently demonstrated an increase in urinary dysfunction rates (Bump, 1998). In those with symptoms, separating aging changes from hypoestrogenism effects is difficult. First, high-affinity estrogen receptors are found in the urethra, pubococcygeal muscle, and bladder trigone but are infrequently found elsewhere in the bladder (Iosif, 1981). Hypoestrogenic-related collagen changes and reductions in urethral vascularity and skeletal muscle volume are factors. They are thought collectively to contribute to impaired urethral function via a decreased resting urethral pressure (Carlile, 1988). Moreover, estrogen deficiency with resulting urogenital atrophy is believed to be responsible in part for urinary sensory symptoms following menopause (Raz, 1993). Despite this current evidence, it is less clear whether estrogen therapy is useful in the treatment or prevention of incontinence. Namely, systemic estrogen replacement, compared with placebo, appears to worsen incontinence, whereas topical vaginal estrogen application may improve incontinence (Cody, 2012; Fantl, 1994, 1996; Rahn, 2014, 2015).
Childbirth and pregnancy also play a role, and urinary incontinence prevalence is higher in parous women compared with nulliparas. The effects of childbirth may result from direct injury to pelvic muscles and connective tissue attachments. In addition, nerve damage from trauma or stretch injury can lead to pelvic muscle dysfunction. Specifically, rates of prolonged pudendal nerve latency after delivery are higher in women with incontinence compared with asymptomatic puerperal women (Snooks, 1986).
Of potential obstetric factors, one large study identified that fetal birthweight >4000 g increased the risk of all urinary incontinence types (Rortveit, 2003b). These authors also noted that cesarean delivery may offer a short-term protective effect from urinary incontinence. The adjusted odds ratio for any incontinence associated with vaginal delivery compared with that with cesarean delivery was 1.7 (Rortveit, 2003a). However, the protective effect of cesarean delivery on incontinence may dissipate after additional deliveries, decreases with age, and is not present in older women (Nygaard, 2006).
Family history may alter incontinence risks, and the urinary incontinence rates may be increased in the daughters and sisters of incontinent women. In one large survey, daughters of incontinent women had an increased relative risk of 1.3 and an absolute risk of 23 percent of having urinary incontinence. Younger sisters of incontinent women also had a greater likelihood of having any urinary incontinence (Hannestad, 2004).
Chronic obstructive pulmonary disease in women older than 60 years significantly increases urinary incontinence risks (Brown, 1996; Diokno, 1990). Similarly, cigarette smoking is identified as an independent risk factor for urinary incontinence. Both current and former smokers have a two- to threefold risk of incontinence compared with nonsmokers (Brown, 1996; Bump, 1992; Diokno, 1990). In one study, investigators found an association between current and former smoking and incontinence, but only for those who smoked more than 20 cigarettes daily. Severe incontinence was weakly associated with smoking regardless of cigarette number (Hannestad, 2003). Theoretically, persistently increased intraabdominal pressures are generated from a smoker’s chronic cough, and collagen synthesis is diminished by smoking’s antiestrogenic effects.
Hysterectomy does not appear to increase urinary incontinence rates. Studies that include pre- and postoperative urodynamic testing reveal clinically insignificant changes in bladder function. Moreover, evidence does not support avoidance of clinically indicated hysterectomy or the selection of supracervical hysterectomy as measures to prevent urinary incontinence (Vervest, 1989; Wake, 1980).
PATHOPHYSIOLOGY
The bladder has the capacity to accommodate large increases in volume with minimal or no increases in intravesical pressure. The ability to store urine coupled with convenient and socially acceptable voluntary emptying is continence. Continence requires the complex coordination of multiple components that include: muscle contraction and relaxation, appropriate connective tissue support, and integrated innervation and communication between these structures. Simplistically, during filling, urethral contraction is coordinated with bladder relaxation and urine is stored. During voiding, the urethra relaxes and the bladder contracts. These mechanisms can be challenged by uninhibited detrusor contractions, marked increases in intraabdominal pressure, and degradation or dysfunction of the various anatomic components of the continence mechanism.
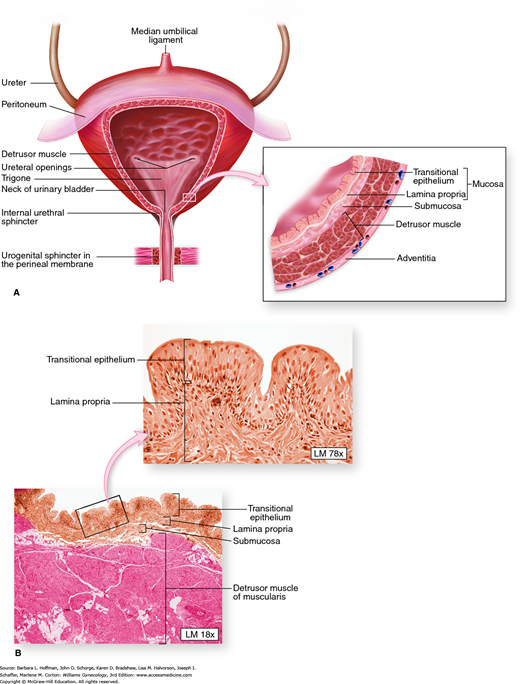
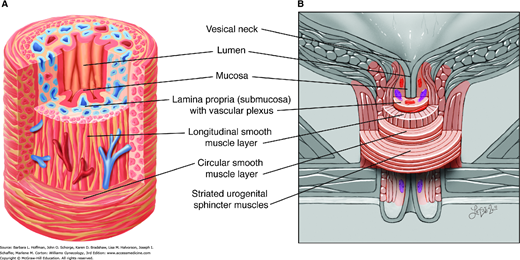
The bladder wall is multilayered and contains mucosal, submucosal, muscular, and adventitial layers (Fig. 23-1). The bladder mucosa is composed of a transitional cell epithelium, supported by a lamina propria. With small bladder volumes, the mucosa appears as convoluted folds. However, with bladder filling, it is stretched and thinned. The bladder epithelium, termed uroepithelium, is made up of distinct cell layers. The most superficial is the umbrella cell layer, and its impermeability is thought to provide the primary urine-plasma barrier. Covering the uroepithelium is a glycosaminoglycan (GAG) layer. This GAG layer may prohibit bacterial adherence and prevents urothelial damage by acting as a protective barrier. Specifically, theories suggest that this carbohydrate polymer layer may be defective in patients with interstitial cystitis (Chap. 11).
FIGURE 23-1
Bladder anatomy. A. Anteroposterior view of bladder anatomy. Inset: The bladder wall contains mucosal, submucosal, muscular, and adventitial layers. B. Photomicrograph of the bladder wall. The mucosa of an empty bladder is thrown into convoluted folds or rugae. The plexiform arrangement of muscle fibers of the detrusor muscle cause difficulty in defining its three distinct layers. (Reproduced with permission from McKinley M, O’Loughlin VD: Human Anatomy. New York: McGraw-Hill; 2006.)
The muscular layer, termed the detrusor muscle, is composed of three smooth-muscle layers arranged in a plexiform fashion. This unique arrangement allows for rapid multidimensional expansion during bladder filling and is a key component to the bladder’s ability to accommodate large volumes.
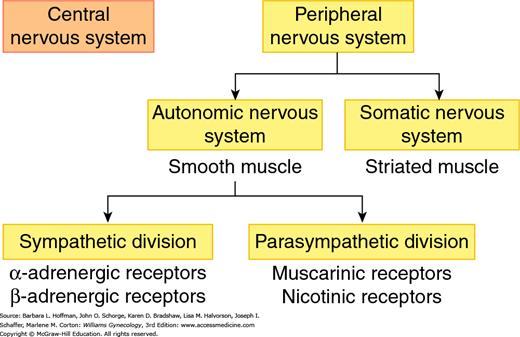
Normal function of the lower urinary tract requires integration of peripheral and central nervous systems. The peripheral nervous system contains somatic and autonomic divisions (Fig. 23-2). Of these, the somatic component innervates striated muscle, whereas the autonomic division innervates smooth muscle.
FIGURE 23-2
Divisions of the human nervous system. The peripheral nervous system includes: (1) the somatic nervous system, which mediates voluntary movements through its actions on striated muscle and (2) the autonomic nervous system, which controls involuntary motion through its actions on smooth muscle. The autonomic nervous system is further divided into the sympathetic division, which acts through epinephrine and norepinephrine binding to adrenergic receptors and (2) the parasympathetic division, which acts through acetylcholine binding to muscarinic or nicotinic receptors.
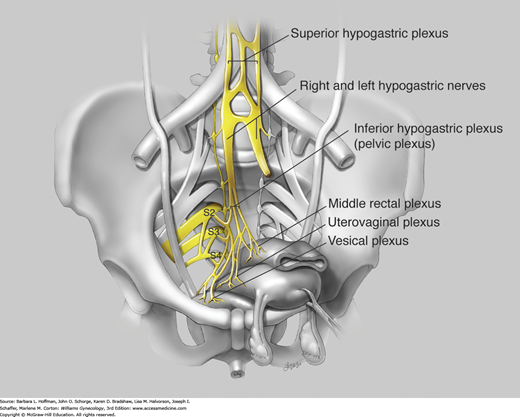
The autonomic nervous system controls involuntary action and is categorized into sympathetic and parasympathetic divisions. The sympathetic system mediates its end-organ effects through epinephrine or norepinephrine acting on α- or β-adrenergic receptors (Fig. 23-3). The parasympathetic division acts through acetylcholine binding to muscarinic or nicotinic receptors. In the pelvis, autonomic fibers that supply the pelvic viscera course in the superior and inferior hypogastric plexi (Fig. 23-4).
FIGURE 23-4
The inferior hypogastric plexus, also known as the pelvic plexus, is formed by visceral efferents from S2 to S4, which provide the parasympathetic component by way of the pelvic nerves. The superior hypogastric plexus primarily contains sympathetic fibers from the T10 to L2 cord segments and terminates by dividing into right and left hypogastric nerves. The hypogastric nerves and rami from the sacral portion of the sympathetic chain contribute the sympathetic component to the pelvic plexus. The pelvic plexus divides into three portions according to the course and distribution of its fibers: the middle rectal plexus, uterovaginal plexus, and vesical plexus. (Used with permission from Lindsay Oksenberg.)
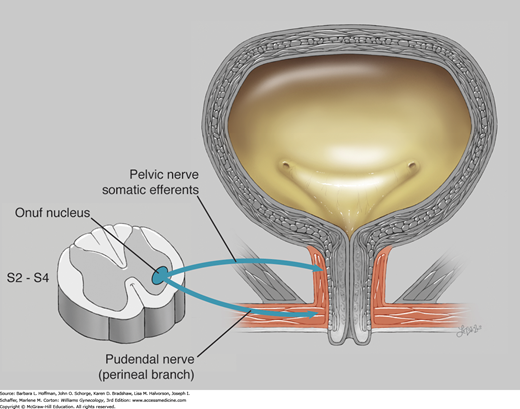
The somatic nervous system controls voluntary movement, and the portion of this system that is most relevant to lower urinary tract function originates from Onuf somatic nucleus. This nucleus is located in the ventral horn gray matter of spinal levels S2–S4 and contains the neurons that innervate the striated urogenital sphincter complex, described next. Nerves involved with that connection include branches of the pudendal and pelvic nerves.
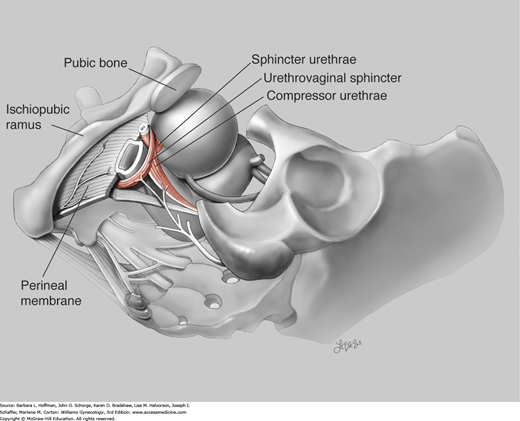
As the bladder fills, synchronized contraction of the urogenital sphincter is integral to continence. Composed of striated muscle, this sphincter complex includes: (1) the sphincter urethrae, (2) the urethrovaginal sphincter, and (3) the compressor urethrae. The sphincter urethrae wraps circumferentially around the urethra. In comparison, the urethrovaginal sphincter and the compressor urethrae arch ventrally over the urethra and insert into the fibromuscular tissue of the anterior vaginal wall (Fig. 23-5).
These three muscles function as a single unit and contract to close the urethra. Contraction of these muscles circumferentially constricts the cephalad two thirds of the urethra and laterally compresses the distal one third. The sphincter urethrae is predominantly composed of slow-twitch fibers and remains tonically contracted, contributing substantially to continence at rest. In contrast, the urethrovaginal sphincter and the compressor urethrae are comprised of fast-twitch muscle fibers, which allow brisk contraction and urethra lumen closure when continence is challenged by sudden increases in intraabdominal pressure.
The urogenital sphincter receives somatic motor innervation through the pudendal and pelvic nerves (Fig. 23-6). Thus, pudendal neuropathy, which may follow obstetric injury, can affect normal sphincter functioning. Additionally, prior pelvic surgery or pelvic radiation therapy may damage nerves, vasculature, and soft tissue. Such injury can lead to ineffective urogenital sphincter action and contribute to incontinence.
FIGURE 23-6
Onuf nucleus is found in the ventral horn gray matter of S2 through S4. This nucleus contains the neurons whose fibers supply the striated urogenital sphincter. The urethrovaginal sphincter and compressor urethrae are innervated by the perineal branch of the pudendal nerve. The sphincter urethrae is variably innervated by somatic efferents that travel in the pelvic nerves. (Used with permission from Lindsay Oksenberg.)
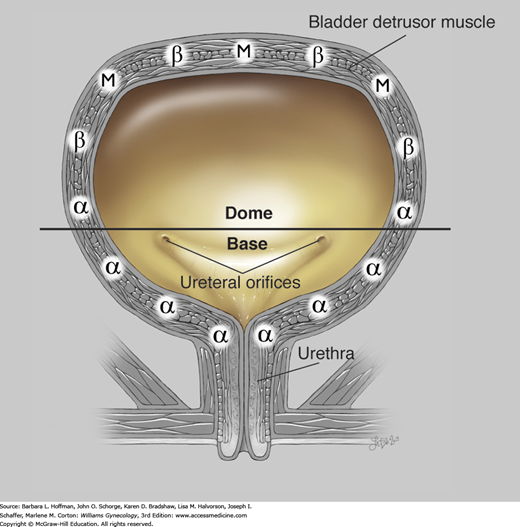
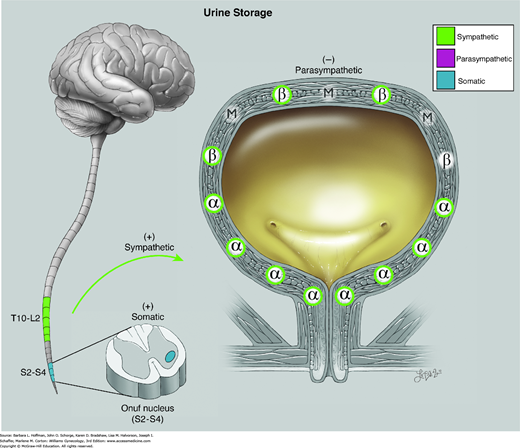
Sympathetic fibers are carried through the superior hypogastric nerve plexus and communicate with α- and β-adrenergic receptors within the bladder and urethra. β-Adrenergic receptor stimulation in the bladder dome results in smooth-muscle relaxation and assists with urine storage (Fig. 23-7). β-Agonist medication may improve overactive bladder symptoms through this mechanism of smooth muscle relaxation. In contrast, α-adrenergic receptors predominate in the bladder base and urethra. These receptors are stimulated by norepinephrine, which initiates a cascade of events that preferentially leads to urethral contraction and aids urine storage and continence. These effects of α-stimulation underlie the treatment of SUI with imipramine, a tricyclic antidepressant with adrenergic agonist properties.
FIGURE 23-7
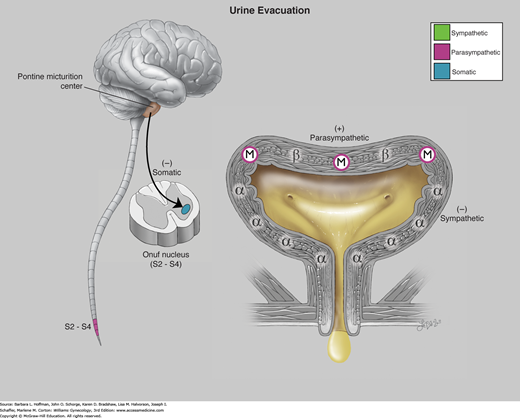
Physiology of urine storage. Bladder distention from filling leads to: (1) α-adrenergic contraction of the urethral smooth muscle and increased tone at the vesical neck (via the T11-L2 spinal sympathetic reflex); (2) activation of urethral motor neurons in Onuf nucleus with contraction of striated urogenital sphincter muscles (via the pudendal nerve); and (3) inhibited parasympathetic transmission with decreased detrusor pressure. α = alpha adrenergic receptors; β = beta adrenergic; M = muscarinic (cholinergic). (Used with permission from Lindsay Oksenberg.)
One key to maintaining continence is adequate urethral mucosal coaptation. The uroepithelium is supported by a connective tissue layer, which is thrown into deep folds, also known as plications. A rich capillary network runs within its subepithelial layer. This vascular network aids in urethral mucosal approximation, also termed coaptation, by acting like an “inflatable cushion” (Fig. 23-8). In women who are hypoestrogenic, this submucosal vasculature plexus is less prominent. In part, hormone replacement targets this diminished vascularity and and in theory, enhances coaptation to improve continence.
FIGURE 23-8
Drawing of urethral anatomy. A. Urethral anatomy in cross section. Urethral coaptation results in part from filling of the rich subepithelial vascular plexus. The urethra contains circular and longitudinal smooth muscle layers. B. Vesical neck and urethral anatomy. The striated urogenital sphincter lies external to the urethral smooth muscle layers. (Used with permission from Lindsay Oksenberg.)
When an appropriate time for bladder emptying arises, sympathetic stimulation is reduced and parasympathetic stimulation is triggered. Specifically, neural impulses carried in the pelvic nerves stimulate acetylcholine release and lead to detrusor muscle contraction (Fig. 23-9). Concurrent with detrusor stimulation, acetylcholine also stimulates muscarinic receptors in the urethra and leads to outlet relaxation for voiding.
FIGURE 23-9
Physiology of urine evacuation. Efferent impulses from the pontine micturition center results in inhibition of somatic fibers in Onuf nucleus and voluntary relaxation of the striated urogenital sphincter muscles. These efferent impulses also result in preganglionic sympathetic inhibition with opening of the vesical neck and parasympathetic stimulation, which results in detrusor muscarinic contraction. The net result is relaxation of the striated urogenital sphincter complex causing decreased urethral pressure, followed almost immediately by detrusor contraction and voiding. α = alpha adrenergic receptors; β = beta adrenergic; M = muscarinic (cholinergic). (Used with permission from Lindsay Oksenberg.)
Within the parasympathetic division, acetylcholine receptors are broadly defined as muscarinic and nicotinic. The bladder is densely supplied with muscarinic receptors, which when stimulated lead to detrusor contraction. Of the muscarinic receptors, five glycoproteins designated M1–M5 have been identified. M2 and M3 receptor subtypes are predominantly responsible for detrusor smooth muscle contraction. Thus, treatment with muscarinic antagonist medication blunts detrusor contraction to improve continence. Continence drugs that target only the M3 receptor maximize drug efficacy yet minimize activation of other muscarinic receptors and drug side effects.
Smooth muscle cells within the detrusor fuse with one another so that a network of low-resistance electrical pathways extends from one muscle cell to the next. Thus, action potentials can spread quickly throughout the detrusor muscle to cause rapid contraction of the entire bladder. In addition, the plexiform arrangement of bladder detrusor fibers allows multidirectional contraction and is ideally suited for rapid concentric contraction during bladder emptying.
During voiding, all components of the striated urogenital sphincter relax. Importantly, bladder contraction and sphincter relaxation must be coordinated for effective voiding. Occasionally, the urethral sphincter fails to relax during contraction of the detrusor and retention ensues. Classically, this is a possible urinary complication of spinal cord injury termed detrusor sphincter dyssynergia and may lead to elevated bladder pressures and vesicoureteral reflux. Women with this condition are sometimes treated with α-blocking agents to help with sphincter relaxation and to lower bladder pressures during contraction, but these may aggravate hypotension. In women without known neurologic pathology but still with inappropriately contracted pelvic floor musculature, treatment with muscle relaxants may be appropriate. These drugs purportedly relax the urethral sphincter and levator ani muscles to improve coordinated voiding.
Theories on continence vary in their supportive evidence but can simplistically be distilled into those that involve anatomic stress incontinence or those that describe decreased urethral integrity (sphincteric deficiency). These theories are not mutually exclusive and in many women, both may be contributory.
First, urethral and bladder neck support is integral to continence. This anatomic support derives from: (1) ligaments along the urethra’s lateral aspects, termed the pubourethral ligaments; (2) the vagina and its lateral fascial condensation; (3) the arcus tendineus fascia pelvis; and (4) levator ani muscles. A full anatomic description of these ligaments and muscles is found in Chapter 38.
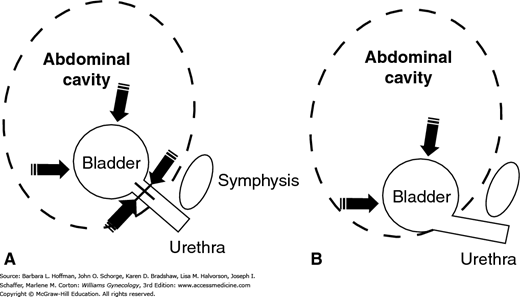
In an ideally supported urogenital tract, increases in intraabdominal pressure are equally transmitted to the bladder, bladder base, and urethra. In women who are continent, increases in downward-directed pressure from cough, laugh, sneeze, and Valsalva maneuver are countered by supportive tissue tone provided by the levator ani muscles and vaginal connective tissue (Fig. 23-10). With loss of support, the ability of the urethra and bladder neck to close against a firm supportive “backboard” is diminished. This results in reduced urethral closing pressures, an inability to resist increases in bladder pressure, and in turn, incontinence. This mechanistic theory is the basis for surgical reestablishment of this support. Traditional procedures such as Burch and Marshall-Marchetti-Kranz (MMK) colposuspensions attempt to return this anatomic support to the urethrovesical junction and proximal urethra.
FIGURE 23-10
Drawing describes the pressure transmission theory. A. In women with normal support, increases in intraabdominal pressure are equally distributed to contralateral sides of the bladder and urethra. B. In those with poor urethral support, increases in intraabdominal pressure alter the urethrovesical angle and continence is lost.
Another way to conceptualize SUI is to consider the urethra as providing continence through the combination of: urethral mucosal coaptation, the underlying urethral vascular plexus, the combined viscous and elastic properties of the urethral epithelium, and contraction of appropriate surrounding musculature. Taken together, these components contribute to urethral integrity. Defects in any or a combination of these components may lead to urine leakage and have traditionally been termed intrinsic sphincteric defect (ISD). For example, prior surgery in the retropubic space may cause denervation and scarring of the urethra and its supporting tissue. These effects subsequently prevent urethral closure and lead to incontinence. Specific causes are varied and include prior pelvic reconstructive surgeries, prior pelvic radiation therapy, diabetic neuropathy, neuronal degenerative diseases, and hypoestrogenism. Namely, in women with atrophic lower genital tracts, vascular changes within the plexus surrounding the urethra lead to poor coaptation and greater incontinence risks.
As noted earlier, nerve dysfunction following birth trauma may lead to defective urethral sphincter function. In addition, childbirth also often injures urethral fascial support. This clinical example highlights the intimate relationship between urethral support and integrity.
Treatments to restore urethral integrity include transurethral injection of bulking agents, surgical sling procedures, and pelvic floor muscle strengthening, which are all described in later sections. In brief, bulking agents are placed at the urethrovesical junction to elevate the epithelium and promote coaptation. Alternatively, sling procedures restore periurethral support anatomy or create partial urethral obstruction to enhance urethral integrity. Last, because the urethra exits through urogenital hiatus, levator ani muscle conditioning with Kegel exercises can bolster urethral integrity. These muscles can be contracted around the urethra when continence is challenged during sudden increases in intraabdominal pressures.
A consideration for surgical management of patients with ISD, particularly those younger than 50 years, is that a retropubic colposuspension procedure merely elevates and stabilizes the urethra and does not promote coaptation. This may be less likely to achieve satisfactory continence than a procedure directed at both anatomic stress incontinence and deficient urethral sphincter function and support (Sand, 1987). That said, a small trial randomizing incontinent women with ISD to Burch or sling procedures did not show differences in postoperative voiding function or in SUI cure rates (Culligan, 2003).
DIAGNOSIS
Assessment of incontinence begins with a patient describing her urinary symptoms. These complaints may be collected through direct conversation but can be augmented with patient questionnaires. Two common forms are the Pelvic Floor Distress Inventory and the Pelvic Floor Impact Questionnaire. Both are available in long and short forms and evaluate urinary, bowel, and prolapse symptoms (Barber, 2001). Such lengthy research questionnaires may be impractical for general clinical practice. Instead, shorter validated questionnaires may easily be incorporated into the clinic setting. As shown in Table 23-2, the 3IQ has only three questions that screen for incontinence and then helps clarify the incontinence type (Brown, 2006).
|
| The response to question 3 with (a) or (b) indicates stress-predominant or urgency-predominant incontinence, respectively, whereas (d) indicates mixed and (c) suggests another cause of incontinence. |
During inquiry, the number of voids and pads used per day, type of pad, frequency of pad changing, and the degree of pad saturation are important. Although these specifics alone may not establish the exact type of incontinence, they do provide information regarding symptom severity and its effects on patient activities. If a woman’s symptoms do not diminish her quality of life, then simple observation is reasonable. Conversely, those with bothersome symptoms warrant further evaluation.
Specific to incontinence, information that describes the circumstances in which urine leaks and specific maneuvers that incite or provoke leakage are sought. With SUI, triggers may include increases in intraabdominal pressure such as coughing, sneezing, Valsalva maneuver, or deep penetration during intercourse. Alternatively, women with urgency urinary incontinence may describe urine loss after urge sensations that typically cannot be suppressed. Overflow incontinence was a term used in the past to refer to women who were unable to empty their bladder well but who also had involuntary, continuous urinary leakage or dribbling and often episodes of incontinence associated with urgency. Currently, however, this is considered by many to reflect another presentation of urgency urinary incontinence.
During questioning, symptoms typically cluster into those most frequently seen with SUI or with urgency urinary incontinence (see Table 23-2). Alternatively, a significant overlap of complaints may reflect coexistent SUI and urgency urinary incontinence, that is, mixed urinary incontinence. For these reasons, pattern identification is helpful as it may direct diagnostic testing and guide initial empiric therapy.
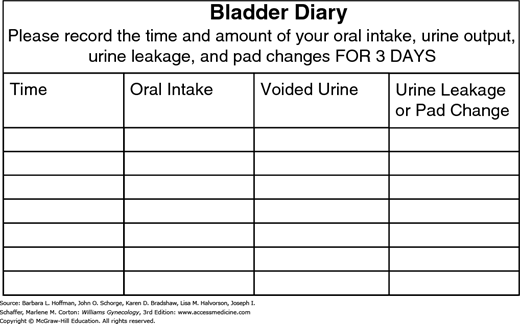
Typically, patients may not have an entirely accurate recollection of their own voiding habits. Accordingly, to obtain a thorough record, a woman ideally completes a urinary diary (Fig. 23-11). With this, the volumes and type of each oral fluid intake, volumes of urine with each void, episodes of urinary leakage, and triggers of incontinence episodes are recorded for 3 to 7 days. During each 24-hour period, women also record times of sleep and awakening to document voluntary nocturnal voiding patterns or enuresis. Three days usually suffices to determine the general trend of incontinence.
The information gained from a voiding/urinary diary is a valuable diagnostic and sometimes therapeutic tool. The first morning void is usually the largest of the day and is a good estimate of bladder capacity. Patients often can identify patterns in intake and voiding and modify behavior. For example, a patient may recognize increased urinary frequency or urgency urinary incontinence episodes after caffeine intake. Moreover, this diary information can serve as a baseline against which treatment effectiveness can be assessed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree