INTRODUCTION
Infertility results from diseases of the reproductive system that impair the body’s ability to perform basic reproductive function. It is defined as the failure to achieve a successful pregnancy after 12 months or more of regular unprotected intercourse. Earlier evaluation and treatment may be justified based on medical history and physical findings and is warranted after 6 months for women older than 35 (American Society for Reproductive Medicine, 2012b). Ten to 15 percent of the reproductive-aged population is infertile, and men and women are equally affected.
Infertility treatment is a complex process influenced by numerous factors. Important considerations include duration of infertility, a couple’s age (especially the female’s), and diagnosed cause. Additionally, the level of distress experienced by a couple should be taken into account.
In general, a first step involves identification of a primary cause and contributing factors, and treatment is aimed at their direct correction. Most are treated with conventional therapies such as medication or surgery. In many cases, therapy can begin without a complete evaluation, especially if a cause is obvious. However, if pregnancy does not quickly follow, then more thorough testing is prudent.
In contrast, evaluation commonly may not yield a satisfactory explanation or may identify causes that are not amenable to direct correction. For such cases, recent advances in assisted reproduction have provided effective treatments. These approaches, however, are not without disadvantage. For example, in vitro fertilization (IVF) has been linked with higher rates of some fetal and maternal complications. Appropriate treatments may also pose ethical dilemmas for couples or their physician. For example, selective reduction of a multifetal pregnancy may improve survival chances for some fetuses but at the cost of others. Last, infertility treatment can be a financial burden, a significant source of emotional stress, or both. During consultation, an infertility specialist does not dictate treatment but offers and explains therapy options, which may include expectant management or even adoption.
LIFESTYLE THERAPIES
Ovarian function is dependent on weight. Low body-fat content is associated with hypothalamic hypogonadism. In contrast, central body fat is associated with insulin resistance and contributes to ovarian dysfunction in many women with polycystic ovarian syndrome (PCOS). Lifestyle modification in overweight infertile women with PCOS leads to a reduction of central fat and improved insulin sensitivity, decreased hyperandrogenemia, lowered luteinizing hormone (LH) concentrations, and restoration of normal fertility in many cases (Hoeger, 2001; Kiddy, 1992). Even a 5 to 10 percent reduction in body weight has been shown to be successful in these women (Crosignani, 2003; Kiddy, 1992; Pasquali, 1989). Apart from diet, exercise can also improve insulin sensitivity. Weight loss and exercise are inexpensive and should be recommended as first-line management of obese women with PCOS.
Although pharmacologic options can effectively treat anovulation if weight cannot be lost, it should be noted that obesity is a significant risk factor for obstetric and perinatal complications. Some maternal risks include higher rates of gestational diabetes, cesarean delivery, preeclampsia, unexplained stillbirth, and surgical wound infection (Cunningham, 2014). Obesity also has been associated with an increased risk of birth defects (American Society for Reproductive Medicine, 2008). Therefore, strong consideration is given to delaying treatments in morbidly obese women until their body mass index (BMI) can be reduced below 40. This is especially true if treatments involve surgical risks or risk of multifetal gestation.
Weight-loss options are discussed in Chapter 1. If bariatric surgery is selected, conception is ideally delayed for 12 to 18 months (American College of Obstetricians and Gynecologists, 2013). This is because rapid weight loss during this time poses theoretical risks for intrauterine fetal-growth restriction and nutritional deprivation.
Undernutrition can also be a problem. The reproductive axis is closely linked to nutritional status, and inhibitory pathways suppress ovulation in subjects with significant weight loss. Anorexia nervosa and bulimia nervosa affect up to 5 percent of reproductive-aged women and may cause amenorrhea, infertility, and in those who do conceive, an increased likelihood of miscarriage. Fortunately, recovery may follow minimal acquisition of weight because energy balance has a more important effect than body fat mass.
Physical activity has numerous health benefits. The relationship between exercise and fertility, however, is not straightforward. Competitive female athletes often experience amenorrhea, irregular cycles or luteal dysfunction, and infertility. This may be related not specifically to physical activity itself but rather to low body-fat content or physical stress associated with competition. At this time, insufficient data exist to support or discourage physical activity in infertile women without documented ovarian dysfunction associated with obesity or low body weight.
In the absence of obesity or significant undernutrition, the role of diet in infertility is unclear. High-protein diets and gluten intolerance (celiac disease) have been investigated as underlying causes in women. However, studies sizes have been small, and conflicting results found (Collin, 1996; Jackson, 2008; Meloni, 1999). In men, dietary antioxidants have been proposed as a potential way to improve male reproductive outcomes by reducing oxidative damage in sperm DNA (Ross, 2010). Although the approach is promising, large well-designed studies to guide its clinical use are needed (Patel, 2008). Additionally, the nutritional supplement carnitine had been often touted as a potential benefit for male infertility. This finding, however, has not been confirmed by a randomized, prospective trial (Sigman, 2006).
Despite a lack of conclusive benefits to nutritional supplements or diet modification in infertile couples, it does seem reasonable to recommend daily multivitamin supplementation to both. Folic acid is contained in most multivitamins, and daily doses of 400 μg orally are recommended for women attempting pregnancy to reduce the incidence of neural-tube defects in their fetuses (American College of Obstetricians and Gynecologist, 2014b).
Herbal therapies including traditional Chinese medicine and acupuncture have been proposed to enhance fertility either alone or in conjunction with standard therapies including assisted reproductive technology (ART). Smith and associates (2010) found that 29 percent of infertile couples seeking pregnancy in the United States had used complementary and alternative medicine. At this time, however, current evidence does not support a benefit of herbal/botanical therapies or acupuncture for fertility as either a primary treatment modality or an adjunct to established therapies (Cheong, 2013).
Stress has been implicated in reproductive failure. Although severe stress can result in anovulation, less significant stress may also play a role, but a mechanism has yet to be defined. Patients with higher stress levels have been found to have lower pregnancy rates when undergoing IVF treatments (Thiering, 1993). Accordingly, screening all infertile couples for evidence of anxiety or depression is a consideration. Although pharmacologic management of stress is not typically recommended during infertility treatments, a “mind/body” approach that combines psychological counseling and meditation may be reasonable for those patients manifesting high levels of anxiety (Domar, 1990).
CORRECTION OF OVARIAN DYSFUNCTION
Prolactin is a pituitary hormone that plays an important role in various reproductive functions, and elevated levels are commonly encountered in clinical endocrinology practice. If hyperprolactinemia is found, then physiologic, pharmacologic, or other secondary causes of hormone hypersecretion are sought (Table 12-2).
Dopamine agonists are the primary treatment of hyperprolactinemia (Chap. 15). Surgical therapies are only considered with prolactin-secreting adenomas resistant to medical therapy. During pregnancy, if hyperprolactinemia is not associated with a pituitary lesion or a lesion is less than 10 mm (microadenoma), then dopamine-agonist therapy is stopped because the tumor expansion risk is low (Molitch, 1999). If the tumor size is 10 mm or larger (macroadenoma), bromocriptine (Parlodel) use is advised during pregnancy to avoid significant tumor growth.
Thyroid disorders are prevalent in reproductive-aged individuals and affect women four to five times more often than men. In women, oligomenorrhea and amenorrhea are frequent findings. Although ovulation and conception can still occur in those with mild hypothyroidism, treatment with thyroxine usually restores a normal menstrual pattern and enhances fertility.
Subclinical hypothyroidism may also be associated with ovarian dysfunction (Strickland, 1990). Lincoln and associates (1999) found a 2-percent incidence of elevated thyroid-stimulating hormone (TSH) levels in 704 asymptomatic women seeking evaluation for infertility. Correction of hypothyroidism in those with ovarian dysfunction and elevated TSH levels led to pregnancy in 64 percent of patients. In addition, subclinical hypothyroidism may also adversely affect pregnancy outcomes, but current evidence does not support that treatment of subclinical hypothyroidism during pregnancy improves these outcomes (Casey, 2014). That said, in women seeking treatment for infertility, early detection and treatment of hypothyroidism of any degree is advised.
Ovarian dysfunction is the most common indication for the use of medications to induce ovulation. These agents can also be selected for ovulatory women to increase the likelihood of pregnancy in couples with other causes of infertility or unexplained infertility. Use of these medications to promote follicular development and prompt ovulation is called superovulation or ovulation enhancement. If these agents are administered solely to stimulate follicles and egg harvesting is completed by ART, then the termed controlled ovarian hyperstimulation is used. In contrast, we prefer the term ovulation induction to describe treatment with medications to stimulate normal ovulation in women with ovarian dysfunction.
Frequent causes of ovarian dysfunction include PCOS and diminished ovarian reserve. Less often, central (hypothalamic or pituitary) disorders or thyroid dysfunction can result in infertility (Table 16-3). Rarely, ovarian tumors or adrenal abnormalities lead to abnormal ovarian function. Treatment of ovarian dysfunction is based on the identified cause and the results of any prior attempted therapy.
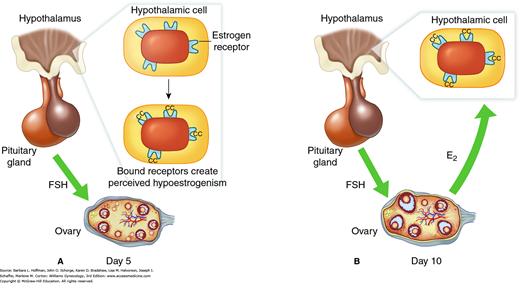
Clomiphene citrate (CC) is the initial treatment for most anovulatory infertile women. Chemically similar to tamoxifen, CC is a nonsteroidal triphenylethylene derivative that demonstrates both estrogen agonist and antagonist properties. Antagonist properties predominate except at very low estrogen levels. As a result, negative feedback that is normally produced by estrogen in the hypothalamus is reduced (Fig. 20-1). Gonadotropin-releasing hormone (GnRH) secretion is altered and stimulates pituitary gonadotropin release. The resulting increase in follicle-stimulating hormone (FSH) levels, in turn, drives ovarian follicular activity.
FIGURE 20-1
Effect of clomiphene citrate (CC) administration. A. Clomiphene binds to the estrogen receptor in the pituitary and hypothalamus. This causes an effective reduction in hypothalamic estrogen receptor number. Because of this reduced receptor number, the hypothalamus and pituitary are effectively blinded to true circulating estrogen levels and perceived hypoestrogenism results. From this, estrogen’s negative feedback is interrupted centrally, and follicle-stimulating hormone (FSH) secretion increases from the anterior pituitary. This leads to maturation of multiple follicles. B. By the late follicular phase, because of clomiphene citrate’s long retention within tissues, estrogen receptor depletion continues centrally. As a result, increased estradiol (E2) secretion from the ovary is not capable of exerting normal negative feedback on FSH release. This leads to a growth of multiple dominant follicles and multiple ovulations.
Tamoxifen has also been used successfully for ovulation induction. However, it is not approved by the Food and Drug Administration (FDA) for this indication and has not been demonstrated to have significant advantage compared with CC.
Clomiphene citrate is administered orally, typically starting on the third to fifth day after the onset of spontaneous or progestin-induced menses. Ovulation rates, conception rates, and pregnancy outcome are similar regardless whether treatment begins on cycle day 2, 3, 4, or 5. Prior to therapy, sonography is advisable to exclude signs of significant spontaneous follicular maturation or residual follicular cysts. In general at our institution, clomiphene can be administered if no follicle is >20 mm and the endometrium is less than 5 mm. A pregnancy test is also indicated after spontaneous menses. Although not a proven teratogen, CC is classified as category X by the FDA and thus is contraindicated in suspected or documented pregnancy.
The dose required to achieve ovulation correlates with body weight. However, there is no reliable way to accurately predict which dose will be required in an individual woman (Lobo, 1982). Consequently, CC is titrated empirically to establish the lowest effective dose for each patient. Treatment typically begins with a single 50-mg tablet taken daily for 5 consecutive days. Doses are increased by a 50-mg increment in each subsequent cycle until ovulation is induced. The dose of CC should not be increased if normal ovulation is confirmed. Thus, lack of pregnancy alone does not justify a dose increase. The effective dose of CC ranges from 50 mg/d to 250 mg/d, although doses in excess of 100 mg/d are not approved by the FDA. Some studies have suggested that adjunctive therapy with glucocorticoids may benefit some patients not responsive to CC alone (Elnashar, 2006; Parsanezhad, 2002). The precise mechanism is unclear, although several direct and indirect actions of dexamethasone have been suggested. This therapy may be empiric or individualized based on elevated dehydroepiandrosterone sulfate (DHEAS) levels.
In general, women failing to ovulate with 100 mg/d dosing or failing to conceive following 3 to 6 months of ovulatory response to CC should be considered candidates for alternative treatments. In a retrospective study including 428 women who received CC for ovulation induction, 84.5 percent of pregnancies achieved with treatment occurred during the first three ovulatory cycles (Gysler, 1982).
Although PCOS appears to be a heterogeneous disorder, many women with this condition exhibit insulin resistance (Chap. 17). Insulin resistance leads to compensatory hyperinsulinemia and dyslipidemia. Given the strong evidence that hyperinsulinemia plays a pivotal pathogenic role in development of PCOS, it is reasonable to assume that interventions that reduce circulating insulin levels in women with PCOS may restore normal reproductive endocrine function. As discussed, weight loss, nutrition, and exercise have clearly led to reduced hyperinsulinemia, resolution of hyperandrogenism, and in some cases, resumption of ovulatory function in overweight women with PCOS. However, women may be poorly compliant, and weight loss is rarely maintained over time.
Insulin-sensitizing agents show promise in the treatment of PCOS. When administered to insulin-resistant patients, these compounds act to increase target tissue responsiveness to insulin, thereby reducing the need for compensatory hyperinsulinemia (Antonucci, 1998). Current insulin-sensitizing agents include the biguanides and thiazolidinediones (Chap. 17).
Of these, studies suggest that metformin (Glucophage), given 500 mg orally three times daily or 850 mg twice daily with meals and administered to women with PCOS, increased the frequency of spontaneous ovulation, menstrual cyclicity, and ovulatory response to CC (Nestler, 1998; Palomba, 2005; Vandermolen, 2001). In contrast, a large, prospective, randomized, multicenter trial does not support the hypothesis that metformin, either alone or in combination with CC, improves the live-birth rate in women with PCOS (Legro, 2007).
Clomiphene citrate is easy to use and leads to ovulation in most patients (Hammond, 1983). However, pregnancy rates are disappointing and approximate ≤50 percent (Raj, 1977; Zarate, 1971). Lower than expected pregnancy rates with CC have been attributed to its long half-life and peripheral antiestrogenic effects, mainly on the endometrium and cervical mucus. For such individuals, who are often classified as “clomiphene resistant,” the next step is traditionally the administration of exogenous gonadotropin preparations via injections.
As with CC, the goal of ovulation induction with gonadotropins is simply to normalize ovarian function. Ideally, the dose used is the minimum required to cause normal development of a single dominant follicle. Because the response to gonadotropins can vary greatly from individual to individual and even from cycle to cycle, intensive monitoring is required to adjust dosage and timing of ovulation.
Gonadotropin preparations vary in terms of their source (urinary or recombinant) and by the presence or absence of LH activity (Table 20-1). Traditional urinary-derived human menopausal gonadotropin (hMG) preparations contain both FSH and LH. These are extracted and purified from the urine of postmenopausal women, in whom FSH and LH levels are normally high. These preparations also contain human chorionic gonadotropin (hCG), which is mainly derived from normal pituitary secretion in postmenopausal women. LH and hCG both bind to the same receptor (luteinizing hormone/chorionic gonadotropin receptor [LHCGR]).
| Name | Product Type | FSH Activity | LH Activity | hCG Activity |
| Bravelle Fertinex a | Vial | Highly purified urinary | Minimal | Minimal |
| Follistim Gonal-f | Pen or vial | Highly purified recombinant | None | None |
| Menopur | Vial | Highly purified urinary | Minimal | Highly purified urinary |
| Repronex Pergonal a Humagon a | Vial | Urinary | Urinary | Urinary |
In contrast, in purified hMG, hCG serves as the primary source of the LH activity, although significant LH is also present in the older, nonhighly purified hMG products (Filicori, 2002). Highly purified urinary preparations allow for administration via subcutaneous route with minimal or no reaction at the injection site. Alternatives to hMG include highly purified urinary gonadotropin preparations and purified recombinant FSH.
Both LH and FSH activity are required for normal ovarian steroidogenesis and follicular development. In many cases, pure FSH preparations can be used because of adequate endogenous LH production. However, for ovulation induction in patients with hypogonadotropic amenorrhea, LH activity must be provided from an exogenous source. Thus, options include hMG, recombinant LH, low-dose (diluted) urinary or recombinant hCG. Ovulation induction in women with PCOS can be performed either with FSH-only containing products or those containing both LH and FSH activity. At present, data do not support the superiority of one preparation over another.
Gonadotropin development will likely continue. A long-acting FSH is commercially available outside the United States. This recombinant molecule was created by adding a DNA sequence to the human FSH gene. This extra sequence allows for more glycosylation and hence a prolonged clearance. Low-molecular-weight molecules (nonproteins) have been identified that activate the FSH and LH receptors. However, these compounds are still in early stages of clinical development. Advantages of these nontraditional gonadotropins include oral delivery.
Most clinicians begin ovulation induction attempts at a low gonadotropin dosage of 50 to 75 IU injected daily. This is gradually increased if no ovarian response (as assessed by serum estradiol measurements) is noted after several days (Fig. 20-2). This is referred to as a “step-up” protocol. A “step-down” protocol can also be used with the advantage of a decreased duration of stimulation. However, the risk of excessive ovarian response, such as multiple follicle development or ovarian hyperstimulation syndrome, may be increased with this method. With either approach, if a patient fails to conceive, subsequent cycles may be started at higher doses based on prior response.
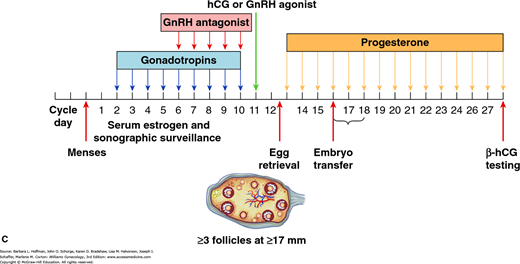
FIGURE 20-2
Drug protocols for ovulation induction. A. Downregulation of gonadotropin-releasing hormone (GnRH) agonist protocol. This is also known as the long protocol. In this diagram, the long protocol is combined with combination oral contraceptive (COC) pill pretreatment. With the long protocol, GnRH agonists are begun typically 7 days prior to gonadotropins. GnRH agonists suppress endogenous pituitary release of gonadotropins. This minimizes the risk of a premature luteinizing hormone (LH) surge and thus premature ovulation. During all protocols, serial serum estrogen levels and sonographic surveillance of follicular development accompany gonadotropin administration. Human chorionic gonadotropin (hCG) is administered to trigger ovulation when sonography shows three or more follicles measuring at least 17 mm. Eggs are retrieved 36 hours later. Embryos are transfer back to the uterus 3–5 days following retrieval. Progesterone supplementation, with either vaginal preparations or intramuscular injection, follows during the luteal phase to support the endometrium. The goal of COC pretreatment is to prevent ovarian cyst formation. One of the major drawbacks of GnRH agonist therapy is the induction of initial transient gonadotropin release or flare, which may lead to ovarian cyst formation. Functional ovarian cysts can prolong the duration of pituitary suppression required prior to gonadotropin initiation and may also exert a detrimental effect on follicular development because of their steroid production. Moreover, COC pretreatment may improve induction results by providing an entire cohort of follicles synchronized at the same developmental stage that will reach maturity at the same time once stimulated by gonadotropins. B. GnRH flare protocol. This is also known as the short protocol. GnRH agonists initially bind gonadotropes and stimulate follicle-stimulating hormone (FSH) and LH release. This initial flare of gonadotropes stimulates follicular development. Following this initial surge of gonadotropins, the GnRH agonist causes receptor downregulation and an ultimately hypogonadotropic state. Gonadotropin injections begin 2 days later to continue follicular growth. As with the long protocol, continued GnRH agonist therapy prevents premature ovulation. C. GnRH antagonist protocol. As with GnRH agonists, these agents are combined with gonadotropins to prevent premature LH surge and ovulation. This protocol attempts to minimize risk of ovarian hyperstimulation syndrome (OHSS) and GnRH side effects, such as hot flashes, headaches, bleeding, and mood changes.
In general, gonadotropin stimulation in women with PCOS is less successful than in patients with hypogonadotropic amenorrhea (Balen, 1994). Women with PCOS have ovaries highly sensitive to gonadotropin stimulation. They have a higher risk of excessive ovarian response and of multifetal pregnancy than those with normal ovaries (Farhi, 1996).
Gonadotropins are associated with more effective ovulation induction and higher pregnancy rates than CC. However, gonadotropins are expensive and carry higher risks for ovarian hyperstimulation syndrome and multifetal gestation. Accordingly, aromatase inhibitors have been investigated as ovulation-inducing agents (Fig. 20-3). These drugs were originally developed for breast cancer treatment and effectively inhibit aromatase, a cytochrome P450 hemoprotein that catalyzes the rate-limiting step in estrogen production. Aromatase inhibitors are orally administered, easy to use, relatively inexpensive, and associated with typically minor side effects (Chap. 10).
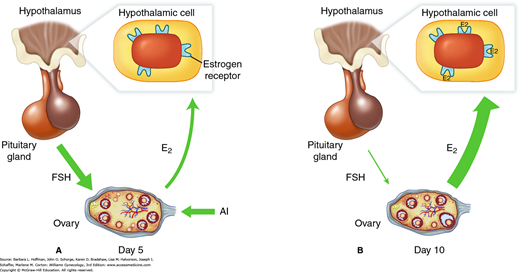
FIGURE 20-3
Effect of aromatase inhibitor (AI) administration. A. Administration suppresses ovarian estradiol (E2) secretion and reduces estrogen negative feedback at the pituitary and hypothalamus. As a result, increased follicle-stimulating hormone (FSH) secretion from the anterior pituitary stimulates growth of multiple ovarian follicles. B. Later in the follicular phase, the effect of the aromatase inhibitor is reduced, and E2 levels increase as a result of follicular growth. Because aromatase inhibitors do not affect estrogen receptors centrally, the increased E2 levels result in normal central negative feedback on FSH secretion. Follicles smaller than the dominant follicle undergo atresia, with resultant monofollicular ovulation in most cases.
The most widely used aromatase inhibitor to induce ovulation in anovulatory and ovulatory infertile women is letrozole (Femara). Compared with CC, its use is associated with higher pregnancy rates following ovulation induction (Legro, 2014). When used in combination with gonadotropins, letrozole leads to lower gonadotropin requirements and may achieve pregnancy rates comparable to gonadotropin treatment alone (Casper, 2003; Mitwally, 2004). The typical dosage used is 2.5 mg to 5 mg orally daily for 5 days.
Data suggesting that letrozole use for infertility treatment might be associated with a higher risk of congenital cardiac and bone malformations in the newborn are contradictory (Biljan, 2005; Tulandi, 2006). However, in 2005, the manufacturer issued a statement to physicians worldwide advising that letrozole use in premenopausal women, specifically its use for ovulation induction, is contraindicated (Fontana, 2005). As a result, it is not likely that letrozole will gain FDA approval or widespread acceptance for ovulation induction in the near future. Larger, well-designed randomized prospective trials that confirm their safety are still needed (Franik, 2014).
A second aromatase inhibitor, anastrozole, is of the same compound class as letrozole and has also been approved for treatment of women with breast cancer. At this time, no concerns have been raised regarding its teratogenicity. However, experience with anastrozole (Arimidex) in ovulation induction at this time is limited, and ideal dosages are currently unknown. Two trials comparing anastrozole to clomiphene have not found it to be more effective than clomiphene (Tredway, 2011a,b).
This is a clinical symptom complex associated with ovarian enlargement resulting from exogenous gonadotropin therapy. Symptoms may include abdominal pain and distention, ascites, gastrointestinal problems, respiratory compromise, oliguria, hemoconcentration, and thromboembolism. These symptoms may develop during ovulation induction or in early pregnancies that were conceived through exogenous ovarian stimulation.
The etiology of ovarian hyperstimulation syndrome (OHSS) is complex, but hCG, either exogenous or endogenous (derived from a resulting pregnancy), is believed to be an early contributing factor. Development of OHSS involves increased vascular permeability and loss of fluid, protein, and electrolytes into the peritoneal cavity, which leads to hemoconcentration. Increased capillary permeability is felt to result from vasoactive substances produced by the corpus luteum. Vascular endothelial growth factor (VEGF) is thought to play a major role, and angiotensin II may also be involved. Hypercoagulability may be related to hyperviscosity following hemoconcentration. Alternatively, it may be secondary to the high estrogen levels present, and these high levels can increase coagulation factor production. Predisposing factors for OHSS include multifollicular ovaries such as with PCOS, young age, high estradiol levels during ovulation induction, and pregnancy.
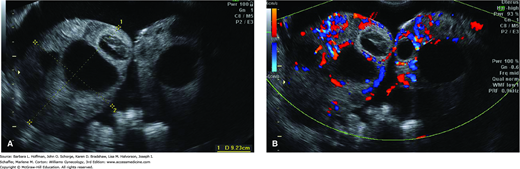
Abdominal pain is prominent and caused by ovarian enlargement and accumulation of peritoneal fluid. Although sonographic examination of women with OHSS usually reveals enlarged ovaries with numerous follicular cysts and ascites, OHSS is a clinical diagnosis (Fig. 20-4). Several different classification schemes have been proposed to categorize the severity of this syndrome, and Table 20-2 lists one.
FIGURE 20-4
A. Sonogram of ovaries with multiple large cysts secondary to ovarian hyperstimulation syndrome. Ovaries are enlarged and meet in the midline. Ascites surrounds these enlarged ovaries. B. Color Doppler transvaginal sonography is often performed to exclude ovarian torsion in these patients.
| Grade 1: | Abdominal distention/discomfort |
| Grade 2: | Grade 1 plus nausea and vomiting or diarrhea |
| Ovaries enlarged 5–12 cm | |
| Grade 3: | Sonographic evidence of ascites |
| Grade 4: | Clinical evidence of ascites or hydrothorax or difficulty breathing |
| Grade 5: | All of the above plus decreased blood volume, hemoconcentration, diminished renal perfusion and function, and coagulation abnormalities |
Treatment of OHSS is generally supportive. Paracentesis is typically performed transvaginally as an outpatient and can ameliorate abdominal discomfort and relieve respiratory distress. Reaccumulation of ascites may prompt additional paracenteses or rarely placement of a percutaneous “pigtail” catheter for continuous drainage. Untreated hypovolemia can lead to renal, hepatic, or pulmonary end-organ failure. Thus, fluid balance must be maintained by replacement with an isotonic fluid such as normal saline. Monitoring of electrolytes is critical. Because of hypercoagulability in these women, prophylaxis for thromboembolism is strongly considered with severe OHSS.
During exogenous ovulation, strategies to avoid OHSS induction include decreasing follicular stimulation (a decreased FSH dose), “coasting” (withholding FSH administration for one or more days prior to the hCG trigger injection), prophylactic treatment with volume expanders, and substitution of hCG for FSH during the final days of ovarian stimulation. With this last strategy, low-dose hCG administration can support maturation of larger ovarian follicles but is postulated to directly or indirectly increase atresia rates of small antral follicles and thereby lower OHSS rates. However, during ovulation induction, if concern for OHSS develops, then the hCG trigger can be withheld, resulting in cycle cancellation. For these patients, IVF should often be considered rather than further attempts at ovulation induction.
OHSS can also develop with ART therapy, and the risk can be substantially reduced with appropriate precautions. Predicted high responders (e.g., high numbers of antral follicles, high AMH level, or prior high response to ovulation induction) should be stimulated with a GnRH antagonist protocol. This allows a single GnRH agonist dose to be used for the “trigger” in place of hCG. The resulting endogenous LH surge can bring about the final stages of follicle and oocyte maturation without significant OHSS risk. Moreover, prevention of pregnancy does not completely eliminate the risk of OHSS but certainly serves to limit symptom duration. Thus, an additional option in ART cycles is to freeze all embryos and forgo embryo transfer that cycle.
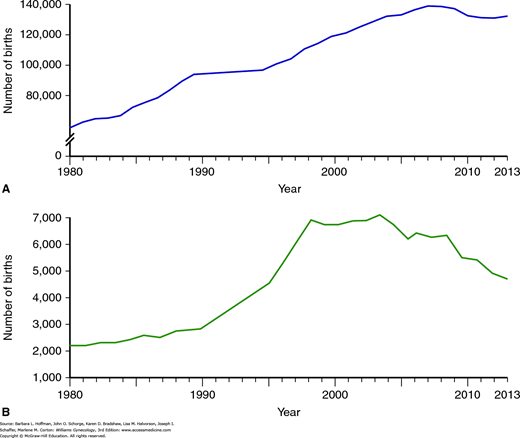
From 1980 through 1997, the number of twin births rose by more than 50 percent, and the number of higher-order multifetal births increased by more than 400 percent (Fig. 20-5) (Martin, 1999). Using data from these years, the Centers for Disease Control and Prevention (CDC) (2000) estimated that approximately 20 percent of triplets and higher-order multifetal births were attributable to spontaneous events; 40 percent were related to ovulation-inducing drugs without ART; and 40 percent resulted from ART. However, further analysis of the same data indicates that the overwhelming majority of all multifetal births result from spontaneously conceived twin gestations and that only approximately 10 percent result from IVF and related procedures.
FIGURE 20-5
Trends in frequency of multifetal gestations. A. Number of twin births in the United States from 1980 to 2006. B. Number of triplet and higher-order multifetal births in the United States for the same time period. (Data from Martin JA, Hamilton BE, Osternman MH, et al: Births: final data for 2013. Natl Vital Stat Rep 64:1, 2015.)
Higher-order multifetal pregnancy is an adverse outcome of infertility treatment. In general, increased fetal number leads to greater risk of perinatal and maternal morbidity and mortality. Prematurity leads to most adverse events in these cases, but fetal-growth restriction and discordance are other potential factors.
Monozygotic gestation is also increased in ovulation induction and ART and is associated with greater fetal risks. These include a three- to fivefold higher perinatal mortality rate compared with that of dizygotic twins. Abnormal placentation also develops at higher rates. Additionally, congenital anomalies are increased two- to threefold in monozygotic twins versus singleton neonates, with an estimated incidence of 10 percent. Initially, extended embryo culture and zona manipulation were postulated to increase the risk of monozygosity. More recent, well-designed trials have refuted this contention (Franasiak, 2015; Papanikolaou, 2010).
Patients with higher-order multifetal gestations are faced with options of continuing their pregnancy with all the risks previously described, terminating the entire pregnancy, or selecting multifetal pregnancy reduction (MFPR). MFPR reduces the number of fetuses to decrease the risk of maternal and perinatal morbidity and mortality. Although MFPR lowers the risks associated with preterm delivery, it often creates profound ethical dilemmas. Moreover, multifetal reduction lowers, but does not eliminate, the risk of fetal-growth restriction in remaining fetuses. With MFPR, pregnancy loss and prematurity are primary risks. However, current data suggest that such complications have decreased as experience with the procedure has grown (Evans, 2008).
Several issues in infertility care contribute to the increased incidence of higher-order multifetal pregnancies. An infertile couple’s sense of urgency may lead to a preference for more aggressive strategies involving gonadotropin treatment or for more embryos to be transferred in IVF cycles. Clinicians may feel competitive pressures to achieve higher pregnancy rates and may be inclined to turn to superovulation or IVF earlier in treatment or to transfer a greater number of embryos.
There have been efforts to lower the rates of multifetal gestation in patients undergoing ovulation induction or superovulation by using serum estradiol limits and arbitrary sonographic criteria of follicular size. These, however, have been ineffective. In a multicenter randomized clinical trial involving 1255 ovulation induction cycles, hCG was withheld if the estradiol concentration rose above 3000 pg/mL or if more than six follicles greater than 18 millimeters in diameter were present (Guzick, 1999). Despite these limits on hCG administration, the multifetal gestation rate was still 30 percent. Although sonography and serum estradiol monitoring have not reduced the incidence of multifetal gestation or OHSS, the risk of multifetal pregnancy does correlate with the magnitude of follicular response as indicated by follicle number and serum estradiol levels. However, there is no consensus among centers regarding specific sonographic criteria or estradiol levels beyond which hCG should not be administered.
When the likelihood of multifetal gestation is felt to be excessive, IVF can be undertaken to reduce the risk. Because the number of embryos transferred can be strictly controlled, this strategy can minimize the risk of higher-order multifetal gestations. Guidelines set forth by the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology (2013a) have led to a significant reduction in triplet (and higher-order) gestations (Table 20-3). Efforts to reduce twin pregnancies through the increased use of elective single embryo transfer (eSET) are currently ongoing (American Society for Reproductive Medicine, 2012c).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree