Cardiorespiratory Adjustments at Birth
Ruben E. Alvaro
▪ INTRODUCTION
Respiratory physiologists and physicians have long been interested in the respiratory and cardiovascular events that occur at birth. However, apart from occasional references to the pulmonary circulation, the fetal circulation only received serious consideration in the middle of the 20th century, when it was recognized that dramatic changes in blood flow through the lungs occurred after birth.
The first detailed description of circulation in the mammalian fetus was provided by Harvey in 1628 (1). Although he correctly described blood flow from the inferior vena cava through the foramen ovale, he thought that the blood had to enter the pulmonary veins before returning to the left atrium. He was also perplexed by how the fetus survives in utero without the aid of respiration. The answer to the last question came in 1799 when Scheel (2) noted light red blood in the umbilical vein and dark red blood in the umbilical artery in the fetal sheep as well as darkening of that color when the pregnant ewe was asphyxiated. However, it was Zweifel (3) in 1876 who categorically stated that the placenta was the lung of the fetus, describing the presence of oxyhemoglobin in the umbilical blood before any breathing had occurred.
The conventional belief during the 19th century was that the fetal pulmonary blood flow progressively increased over gestation and that it was relatively higher in the fetus than after birth (4,5). It was not until the first part of the 20th century that the right ventricular pressure was demonstrated to fall and pulmonary blood flow to increase after the establishment of breathing (6,7). It was only 60 years ago that Dawes et al. (8) demonstrated by direct measurements in fetal lambs that pulmonary blood flow increased when the lungs were ventilated with air. Over these past 60 years, the developmental changes in the pulmonary circulation and its responses to hypoxia, increases in pulmonary arterial pressure and blood flow, have become subjects of intense investigation (9).
The transition from the placenta to the lungs at birth is accomplished by three main cardiopulmonary processes:
Onset of breathing, resulting in lung expansion with concomitant decrease in pulmonary vascular resistance (PVR) and increase in pulmonary blood flow
Increase in blood oxygen content further decreasing PVR
Loss of the placental circulation with resultant increase in systemic vascular resistance leading to the closure of the fetal cardiovascular shunts and transition from fetal to neonatal circulation
Thus, to establish the lungs as the site of gas exchange after birth, significant changes in the cardiac and pulmonary circulation as well as the initiation of pulmonary ventilation must occur. Other essential adaptations to extrauterine life are changes in endocrine function, substrate metabolism, and thermogenesis. Many abnormal maternal, placental, and fetal conditions may interfere with this physiologic transition and compromise the newborn infant.
The establishment of effective pulmonary ventilation at birth requires that the lungs develop to a stage where the alveoli can be inflated to provide adequate gas exchange. It also requires the lowering of the PVR to allow for the increase in pulmonary blood flow to accommodate the entire cardiac output. The successful transition also requires that the lung liquid volume be removed from the alveolar spaces and that surface active material be secreted into the acinus to allow for satisfactory and sustained physical expansion of the lungs after the initial postnatal breaths. Adequate neurologic drive to generate and maintain spontaneous continuous breathing is essential to maintain ventilation postnatally.
In this chapter, we review some of the most important cardio-respiratory adjustments that occur at the time of delivery allowing the fetus to achieve a successful extrauterine transition
▪ PULMONARY ADAPTATION
Fetal Lung Fluid
During fetal life, the internal volume of the lungs is maintained by the secretion of liquid into the pulmonary lumen. This liquid expansion of potential air spaces is essential for the growth and the development of normal lung structure before birth, which in turn may influence lung function after birth (10).
The fluid in the fetal lung was for many years assumed to be aspirated amniotic fluid as a result of fetal breathing movements (11). In 1941, Potter and Bohlender (12) observed alveolar fluid in two human fetuses with malformations of the respiratory tract that blocked the entrance of amniotic fluid, thus establishing that the lung fluid was secreted, not inhaled. Experiments performed in other species confirmed that fetal pulmonary fluid was indeed generated within the lungs (13,14,15).
We know now that the fetal lung fluid is neither a mere ultrafiltrate of plasma nor aspirated amniotic fluid. Compared to plasma, this lung fluid is rich in chloride and potassium, is significantly lower in bicarbonate, and has similar sodium concentration. It is also quite different from amniotic fluid having much higher osmolality, Na+ and Cl− concentrations, and significantly lower K+, protein, and urea concentration (Table 16.1) (10,16,17,18). This distinctive composition of the lung liquid changes very little during gestation (17,19). The high Cl− and the low protein content characteristics of the lung fluid result from active Cl− secretion and tight junctions between epithelial cells, respectively.
It is not known exactly when this secretory activity begins, but already during the glandular stage of lung development at about 3 months of gestation, the lung epithelium actively secretes fluid (20,21). By this time, the epithelium has developed tight junctions that are evident by morphologic examination and also by the low protein concentration present in the lung liquid in relationship to the plasma. In fetal lambs, the volume of lung liquid increases from about 5 mL/kg of body weight at midgestation (18) to about 30 to 50 mL/kg at term (18,22,23,24,25). The secretion rate increases from
about 2 mL/kg body weight at midgestation (18) to about 5 mL/kg at term (25,26). The lung liquid secretion decreases with increased luminal hydrostatic pressure induced by a prolonged obstruction of the fetal trachea and increases when luminal pressure falls below amniotic fluid pressure and when fetal breathing movements are abolished (24).
about 2 mL/kg body weight at midgestation (18) to about 5 mL/kg at term (25,26). The lung liquid secretion decreases with increased luminal hydrostatic pressure induced by a prolonged obstruction of the fetal trachea and increases when luminal pressure falls below amniotic fluid pressure and when fetal breathing movements are abolished (24).
TABLE 16.1 Composition of Lung Luminal Liquid, Amniotic Liquid, and Plasma of Fetal Lambs | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
This liquid secreted by the fetal lungs flows intermittently up the trachea with fetal breathing movements. Some of this fluid is swallowed, and the remainder contributes directly to the formation of amniotic fluid production accounting for approximately 25% to 50% of the amniotic fluid turnover in the sheep fetus, the remainder being formed by the fetal urine (10). The mechanism by which amniotic fluid is not aspirated into the lungs was demonstrated by Brown et al. (27) in 1983, when they showed that the larynx acts as a one-way valve allowing only liquid outflow under normal circumstances. The continuous secretion of liquid by the lungs confronted with a flow impediment produced by the larynx and the amniotic fluid pressure creates a small but important positive intrapulmonary pressure, which is essential for normal growth and for the structural and biochemical maturation of the developing lung (10,27,28). Thus, in fetal sheep, unimpeded leakage of tracheal liquid decreases lung size by arresting pulmonary tissue growth, whereas prolonged obstruction of tracheal outflow leads to lung hyperplasia (28,29). Nardo et al. (30) have shown that lung hypoplasia in fetal sheep can be considerably improved by short-term obstruction at the tracheal level. On the other hand, pulmonary hypoplasia in humans can be observed in pathologic conditions such as diaphragmatic hernia, pleural effusion, or severe oligohydramnios (Potter syndrome) as a result of the compression of the fetal lungs and the decrease in their internal volume (31,32).
Congenital high airway obstruction syndrome (CHAOS) is a clinical condition caused by complete or near-complete obstruction of the fetal airway that results in elevated intratracheal pressure, distension of the tracheobronchial tree, and lung hyperplasia. The enlarged lungs may cause cardiac and caval compression leading to in utero heart failure manifested by ascites, hydrops fetalis, and placentomegaly (33,34).
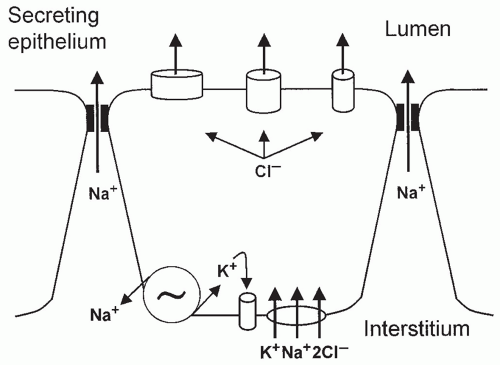
The production of fetal lung liquid depends on a system of active ion transport across the alveolar type II cells of the pulmonary epithelium (10,35,36). Olver and Strang (37) demonstrated that lung liquid secretion is coupled with active transport of Cl− toward the pulmonary lumen, generating an electrical potential difference of -5 mV (lumen negative). This chloride secretion generates an osmotic gradient that causes liquid to flow from the microcirculation through the interstitium into the potential air spaces. This chloride secretion occurs through chloride channels in the apical membrane (alveolar side) and depends largely on chloride influx at a bumetanide-sensitive Na+-K+-2Cl− (NKCC) cotransporter system in the basolateral membrane (interstitial side) (38). Thus, Cl− enters the cell on a cotransporter linked with K+ and Na+ down the electrochemical potential gradient for Na+ generated by Na+-K+-ATPase in the basolateral membrane of the cell. Consequently, Cl− concentration increases inside the cell, above its equilibrium potential, which provides an electrochemical gradient for Cl− exit across the luminal membrane of the epithelial cell through Cl− permeant ion channels (Fig. 16.1) (10). Addition of the loop diuretic bumetanide or furosemide (specific NKCC inhibitors) into the fetal lung liquid decreases fluid secretion by decreasing Cl− entry into the epithelial cell through the basolateral membrane.
Clearance of Fluid at Birth
Pulmonary fluid, essential to fetal lung development, must be rapidly removed at birth in order to allow adequate postnatal gas exchange (39,40,41). Thus, the transition from intra- to extrauterine life requires the effective clearance of lung liquid to support air breathing and the conversion of the pulmonary epithelium in the distal air spaces from fluid secretion to fluid absorption. Disruption of this process has been implicated in several disease states, including transient tachypnea of the newborn (TTN) and hyaline membrane disease (HMD) (40,42,43,44). Preterm delivery and cesarean section without prior labor result in excessive retention of lung fluid and may contribute to respiratory compromise in the newborn infant (43,45,46,47,48,49).
Although a complete understanding is still lacking, it is now clear that the mechanisms by which fetal lungs are able to clear themselves of fluid at birth are multidimensional and can occur via a number of processes before, during, and after birth (50).
Airway Clearance Before Birth
It is well known now that net alveolar fluid clearance occurs at a rapid rate late in gestation and that this clearance is driven by elevations of endogenous epinephrine. The critical link between β-adrenergic stimulation and lung fluid clearance was made in 1978 by Walters and Olver (51) who found that intravenously infused epinephrine caused rapid absorption of lung fluid in near-term fetal lambs and that this response could be inhibited by prior treatment with
propanolol. The intravenous epinephrine caused an immediate and reversible increase in luminal electronegativity by stimulating the active transport of Na+ out of the lung lumen (52). In the absence of adrenergic stimulation, the amiloride-sensitive Na+ channels remain closed (secretory state). Thus, the opened or closed state of the Na+ channels determines whether the fetal lungs, at any particular time, are secretory or reabsorptive (10). The induced reabsorption with epinephrine increases strikingly with advancing gestational age and can be induced by pretreatment of fetal sheep with the combination of corticosteroids and triiodothyronine (27). These two hormones are required to switch the effect of β-adrenergic stimulation from net chloride and liquid secretion to net sodium and liquid absorption (53,54,55,56). Culture, animal, and human studies have shown that activation of sodium channels (ENaCs) by increased circulating levels of adrenaline and vasopressin are important mechanisms of lung liquid reabsorption during labor (10,27,40,52,53,54,57).
propanolol. The intravenous epinephrine caused an immediate and reversible increase in luminal electronegativity by stimulating the active transport of Na+ out of the lung lumen (52). In the absence of adrenergic stimulation, the amiloride-sensitive Na+ channels remain closed (secretory state). Thus, the opened or closed state of the Na+ channels determines whether the fetal lungs, at any particular time, are secretory or reabsorptive (10). The induced reabsorption with epinephrine increases strikingly with advancing gestational age and can be induced by pretreatment of fetal sheep with the combination of corticosteroids and triiodothyronine (27). These two hormones are required to switch the effect of β-adrenergic stimulation from net chloride and liquid secretion to net sodium and liquid absorption (53,54,55,56). Culture, animal, and human studies have shown that activation of sodium channels (ENaCs) by increased circulating levels of adrenaline and vasopressin are important mechanisms of lung liquid reabsorption during labor (10,27,40,52,53,54,57).
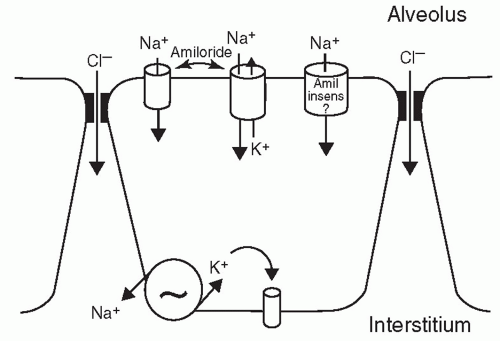
The movement of sodium across the pulmonary epithelium from the alveolar lumen to the interstitium with subsequent absorption into the vasculature can be considered a two-step process. In the first step, sodium passively enters the apical membrane of the alveolar type II cell, through amiloride-sensitive Na+ channels (ENaC). In the second step, sodium is actively pumped out of the cell into the interstitium through the basolateral membrane by the ouabain inhibitable Na+-K+-ATPase. Thus, Na+-K+-ATPase pump inhibition with ouabain consistently reduces liquid clearance in various species (58,59,60,61,62,63). To equilibrate the osmotic pressure, generated by the movement of Na+, water diffuses from the alveolar to the interstitial space either through specific water channels (aquaporins) or through the paracellular junctions (Fig. 16.2) (40,64,65). The gene for amiloride-sensitive epithelial sodium channel was cloned and consists of three homologous subunits called α-, β-, and γ-ENaC. The α-subunit is a prerequisite unit for any channel activity. The expression of the three subunits is necessary for maximum ion transport (66). The three subunits increase sharply around the time of birth, reaching a peak and then decline in parallel with endogenous plasma epinephrine concentration during the 1st week of life.
Another factor that appears to be particularly important in the switch of transepithelial liquid flow from secretion to absorption is the sharp increase in alveolar PO2 that occurs at birth. It was recently shown that fetal PO2 favored the development of fluid-filled cystlike structures while the rise of PO2 to postnatal levels reduced fluid in the cysts (67). The effect of oxygen seems to be the result of an increase in Na+-K+-ATPase activity, and the response is also enhanced by glucocorticoids and thyroid hormones. Thus, at birth, epinephrine, oxygen, glucocorticoid, and thyroid hormones interact to produce a permanent switch from secretion to absorption in the distal epithelium.
Airway Clearance after Birth
Until recently, activation of pulmonary epithelial sodium channels was thought to be the primary mechanism responsible for airway liquid clearance at birth promoting liquid reabsorption by reversing the osmotic gradient across the pulmonary epithelium. However, ENaC activation is unlikely to be the only mechanism of airway liquid clearance at birth for several reasons. First, since effective gas exchange occurs within seconds to minutes of birth, clearance of lung liquid fluid needs to take place very fast. However, animal studies have shown that reabsorption of airway fluid by activation of ENaC receptors by pharmacologic doses of adrenaline takes several hours (51,68). Recent studies using x-ray imaging have demonstrated that lung aeration occurs at a rate of approximately 3 mL/kg/s during inspiration, which is considerably greater than can be achieved with adrenaline (approximately 0.003 mL/kg/s) (51,68,69,70). Second, deletion of β- and γ-ENaC subunits did not cause respiratory failure in newborn pups, despite reducing ENaC activity sixfold (71). Furthermore, Bonny and Rossier (72) have recently described infants with significantly reduced ENaC activity due to gene mutations that do not exhibit neonatal respiratory failure. Third, animal studies have shown that inhibition of epithelial sodium transport by amiloride delays but does not block airway liquid clearance at birth (73).
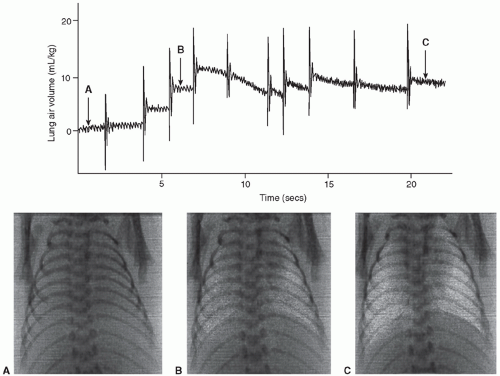
Recent studies in rabbit pups using time-lapsed phase contrast x-ray imaging have demonstrated that transpulmonary hydrostatic pressures generated during inspiration play a major role in airway liquid clearance and FRC development at birth (69,70). These studies have shown that FRC accumulates with each breath and the FRC volume increase equals the volume of liquid leaving the airways resulting in a “steplike” increase in FRC with each breath (Fig. 16.3) (69,70). The same research group recently observed that the FRC development was not dependent on ENaC activity and that increased airway pressures can compensate for the absence of ENaC activity and facilitate liquid clearing. In this study, although inhibition of ENaC activity with amiloride had little effect on lung aeration, it produced a greater FRC loss between inflations. This suggests that ENaC activity by generating an osmotic gradient that opposes the elevated interstitial tissue pressure caused by liquid accumulation within the interstitial tissue compartment at end expiration prevents liquid reentry into the air spaces when transpulmonary hydrostatic pressure gradients were low (74).
Thus, air inflation, by passive reabsorption down the transpulmonary pressure gradient associated with ventilation, shifts liquid from the lung lumen into the interstitium around distensible perivascular spaces of large pulmonary blood vessels and airways. These perivascular cuffs progressively diminish in size as the fluid is removed by small pulmonary blood vessels and lymphatics. Bland et al. (75) have shown that the pulmonary circulation absorbs most of the residual liquid present in potential air spaces at birth and that elevated left atrial pressure or reduction of plasma protein concentration slows the rate of liquid clearance in mature animals (75,76,77).
▪ RESPIRATORY ADAPTATION
Fetal Breathing
The discovery of fetal breathing in the late 1960s immediately stimulated interest in the factors that control breathing in utero (78,79,80). Although of unknown purpose, since no gas exchange is involved, fetal breathing may represent preparation in utero for a vital function important in life. Shortly after its discovery, the Oxford group showed that, although fetal breathing was influenced by fetal behavior, occurring essentially in rapid eye movements (REMs) sleep, it was clearly regulated by other chemical factors,
such as carbon dioxide and oxygen concentration (81). Subsequent work confirmed and expanded these findings by recording the electrical activity of the diaphragm and clearly demonstrating the central origin of the respiratory output in utero (82,83,84,85,86). Using ultrasound technology, breathing movements were also identified in the human fetus, being present about 40% of the time during late pregnancy, a figure similar to that in sheep (78,87,88,89).
such as carbon dioxide and oxygen concentration (81). Subsequent work confirmed and expanded these findings by recording the electrical activity of the diaphragm and clearly demonstrating the central origin of the respiratory output in utero (82,83,84,85,86). Using ultrasound technology, breathing movements were also identified in the human fetus, being present about 40% of the time during late pregnancy, a figure similar to that in sheep (78,87,88,89).
The discovery of fetal breathing not only stimulated the development of the area of fetal assessment but also brought a new dimension to the events occurring at birth. What has been traditionally called “the initiation of breathing at birth” must now be called “the establishment of continuous breathing at birth.” Breathing begins long before birth. The question is not what determines the appearance of breathing at birth, but what makes it continuous. From another angle, what makes fetal breathing episodic in late gestation and present only during low-voltage electrocortical activity? The answer to this question remains unknown.
Fetal breathing in sheep is mostly continuous in early gestation (90 to 115 days) but becomes episodic in late gestation, primarily occurring during periods of low-voltage electrocortical activity (79,80,86,90,91). During high-voltage electrocortical activity, there is no established breathing present, but occasional breaths may surface after episodic, generalized, tonic muscular discharges associated with body movements (Fig. 16.4) (86). During low-voltage electrocortical activity, breathing is irregular, the diaphragmatic electromyography (EMG) being characterized by an abrupt beginning and end. The physiologic mechanism responsible for the occurrence of fetal breathing only during low-voltage electrocortical activity is unknown.
Many studies have clearly shown that the fetal breathing apparatus is capable of responding well to chemical stimuli and other agents known to modify breathing postnatally. Thus, it became clear that the fetus responds to an increase in PaCO2 with an increase in breathing (81,87,92,93,94,95,96). The increased breathing activity is prolonged into the transitional high-voltage electrocortical activity (ECoG) but does not continue into the established high-voltage ECoG (Fig. 16.5). There is much evidence suggesting that the actions of CO2 are central.
Administration of low oxygen to the fetus by having the ewe breathe hypoxic mixtures abolished fetal breathing; this was associated with a decrease in body movements and in the amplitude of the ECoG (81,97,98). Transection of the brain at the upper level of the pons prevents the inhibitory action of hypoxia and induces continuous breathing. Conversely, increase in arterial PO2 to levels above 200 Torr through the administration of 100% O2 to the fetus via an endotracheal tube stimulated breathing and induced continuous breathing in 35% of the experiments in fetal sheep (99). These findings suggest that low partial tension of O2 in the fetus at rest may be a normal mechanism inhibiting breathing in utero.
Establishment of Continuous Breathing at Birth
The traditional view has been that labor and delivery produce a transient fetal asphyxia, which stimulates the peripheral chemoreceptors to induce the first breath. Breathing would then be maintained
through the input of other stimuli, such as cold or touch (100,101). More recent observations have questioned this general view. First, the denervation of the carotid and aortic chemoreceptors does not alter fetal breathing or the initiation of continuous breathing at birth (102



through the input of other stimuli, such as cold or touch (100,101). More recent observations have questioned this general view. First, the denervation of the carotid and aortic chemoreceptors does not alter fetal breathing or the initiation of continuous breathing at birth (102
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree