Human milk is a living, dynamic, adaptive fluid. It contains over 200 known components, including live lymphocytes, macrophages, and neutrophils; immunoglobulins, complement, oligosaccharides, and other host defense factors; lactoferrin; enzymes; and hormones such as corticosteroids, erythropoietin, and insulin to name a few, in addition to its nutrients. There are complex interactions between these components, which likely enhance and contribute to their functions. Human milk is ever adapting, from the beginning to the end of a feeding session, throughout the course of the day, and over the course of lactation. It can never be replicated, no matter how much advertising to the contrary. Adding a “new” component to artificial formula because it is present in human milk does not in any way guarantee that its function or performance will be identical. Human milk has changed and adapted over the course of human evolution to provide exactly what human infants need. It differs from the milk of other mammalian species, including bovines, which have concomitantly evolved to provide what the young of each of those species require to optimally grow and mature.
Accepting that human milk is the species-specific standard in nutrition for human babies, the outcomes associated with its use are then the norm to which other forms of nutrition are compared. That being the case, instead of a discussion of the “benefits of breastfeeding,” it is more accurate to look at the “risks of artificial feeding.” There is a large evidence-based body of literature detailing improved health and developmental outcomes of human milk-fed
term babies and their mothers, over artificially fed babies, which have been well reviewed elsewhere (
1,
2,
3,
4,
5,
32,
33). The economic savings to families, health care payers, employers, and society have also been studied. A cost analysis published in 2010 for all pediatric diseases for which the Agency for Healthcare Research and Quality reported risk ratios that favored breastfeeding, revealed that if 90% of U.S. families could comply with medical recommendations to breastfeed exclusively for 6 months, the United States would save $13 billion per year and prevent an excess 911 deaths, nearly all of which would be in infants (
34). In a 2013 analysis assuming observed associations between breastfeeding duration and maternal health are causal, it was estimated that current U.S. breastfeeding rates result in 4,981 excess cases of breast cancer, 53,847 cases of hypertension, and 13,946 cases of myocardial infarction per year in the population of women who did not breastfeed their offspring, compared with a cohort of 1.88 million U.S. women who optimally breastfed. Suboptimal breastfeeding was calculated to incur a total of $17.4 billion in cost to society resulting from premature death, plus $733.7 million in direct costs, and $126.1 million indirect morbidity costs (
35). These disadvantages of using artificial formulas are summarized in
Table 21.2.
There is every reason to assume that these same advantages of human milk to term, healthy babies and their mothers also apply to preterm and sick neonates and their mothers. Additionally, there is increasing research-based evidence of both short- and long-term positive effects on prematurity-related conditions, including nutrition, gastrointestinal (GI) function, host defense, neurodevelopment, and physiologic well-being.
Nutritional Advantages
The AAP (
1) strongly recommends that human milk is the preferred nutrition not only for healthy term infants but for all infants, including premature and sick newborns, with rare exceptions (
1). For an excellent in-depth examination of this topic, see
Chapter 20. The reader is also referred to a recent review article on the use of human milk for premature infants (
36).
There are, however, several points that are worth reiterating here. Preterm milk differs significantly from term milk (
Table 21.3). Notably, preterm milk has higher concentrations of protein, fatty acids, sodium, and chloride (
37,
38), which, interestingly, are all components required in higher amounts by babies born early. This phenomenon was initially attributed to lower milk volumes produced by mothers of preterm babies, thus causing a concentrating effect on these nutrients. However, contrary to this theory, other components of preterm milk are present in the same concentrations as in term milk. It has subsequently been shown that preterm milk has similar volumes to term milk. Some initially speculated that this is a maternal adaptation to the delivery of her premature infant, although these differences are likely the end result of the interruption in maturation of the mammary gland during pregnancy. In a study looking at total nitrogen, fat, lactose, and carbohydrate concentrations in human milk, GA at birth was inversely related to carbohydrate concentration; postmenstrual age (PMA; an indicator of autonomous developmental processes not affected by the moment of birth) was not related to milk composition; and postnatal age (PNA) was related to a decrease in total nitrogen and an increase in lactose concentration. These data were interpreted to indicate that PNA strongly influences the development of the composition of very preterm human milk, GA affects carbohydrate content with a negligible effect on the nutritional value of the milk, and PMA has no effect (
39) consistent with changes in maturation of the mammary gland.
The higher concentration of nutrients in preterm human milk decrease to approximately term milk levels over the course of the first postnatal month, regardless of the GA of the baby at delivery, although the premature baby’s increased needs continue until approximately term corrected GA. With the advantage of the earlier higher concentrations, particularly of protein and electrolytes, that premature milk affords initially no longer present, we are often required to fortify human milk for the smallest babies after reaching adequate volumes. Babies less than 1,500 g have been shown to require fortification with additional calories, protein, calcium, phosphorus, sodium chloride, and some vitamins to preclude poor growth rates, hyponatremia, hypochloremia, and osteopenia (
36). Larger, more mature babies thrive on mother’s milk alone. Because human milk content differs not only over the course of lactation but also during the course of a feed or milk expression session, at different times of the day, and for those babies requiring feeding by alternative methods, by the method used, it is critical to monitor these VLBW babies for growth rates, serum sodium levels, and bone mineralization status (see also
Chapters 20 and
33). Recent
discussions have suggested that due to variations throughout the day in some human milk components, it might be prudent to have mothers collect their milk in 24-hour aliquots, which, when gently mixed, would theoretically yield a less variable feed. This has yet to be studied.
Lipids
Human milk lipids have sparked great interest in milk components, with the increasing body of literature on the positive neurodevelopmental effects of the long-chain polyunsaturated fatty acids (LC-PUFAs), in particular docosahexaenoic acid (DHA) and arachidonic acid (AA). They are found in phospholipids in the brain, retina, and red blood cell membranes. These LC-PUFAs are not readily synthesized by preterm babies and are normally delivered via the placenta throughout the third trimester. They occur naturally in human milk but are not found in bovine milk. For premature babies, LC-PUFAs must be delivered via an external source, in this case easily by human milk. Because of studies that show improved neurodevelopmental outcome and visual function in breastfed preterm babies and in formula-fed preterm babies supplemented with exogenous sources of LC-PUFAs, DHA and AA have now been added commercially to most term and preterm infant formulas. Of concern for ultimate function is that these additives are of plant origin and are structurally different than human LC-PUFAs (see “
Neurodevelopmental Advantages”).
Human milk lipids are an easily digested source of energy, in part as a result of their composition and in part as a result of their packaging with bile salt-stimulated lipases present in the milk, providing approximately 50% of the total milk calories. Additionally, they provide cholesterol, which is an essential component of membranes. Human milk-fed babies show significantly higher plasma cholesterol levels than do formula-fed or mixed-fed babies (
41). Although one might then expect human milk-fed babies to have higher cholesterol levels than do formula-fed babies as young adults, the opposite has been found to be true, in addition to lower C-reactive protein, a measure of the inflammatory process associated with atherosclerosis (
42). Additionally, one study has shown men who were breastfed as infants to have better endothelial function in young adulthood than did men who were formula fed by showing that breastfeeding was inversely associated with atherosclerosis, measured by the thickness of the intima-media, arterial distensibility, or the prevalence of carotid plaques (
43). It has been postulated that this early exogenous exposure to cholesterol, a necessary nutrient, keeps endogenous cholesterol production downregulated, thus resulting in lower cholesterol levels in adult life. The mechanisms remain to be elucidated.
Gastrointestinal Advantages
In addition to the species specificity and superior digestibility of the nutrients in human milk for premature babies, human milk also favorably affects the function and maturation of the GI tract. Human milk has been shown
in vivo to decrease intestinal permeability in preterm infants when compared to preterm formula (
50). Several studies have found that human milk promotes more rapid
gastric emptying than does artificial formula (
51,
52), with one study revealing that on average, human milk emptied twice as fast as formula (
52). This has implications for clinical practice. Delayed gastric emptying, which generally presents clinically as measurable gastric residuals or vomiting, prevents advancement of enteral nutrition. Babies who cannot reach full enteral feeds require longer periods of parenteral nutrition, with the concomitant risks inherent in both prolonged intravenous catheter usage (e.g., infection, thrombosis, chemical infiltrates) and prolonged intravenous nutrition (e.g., mineral or electrolyte imbalance, hepatic damage). Any of these can impact length of stay (LOS), which in turn has economic and social/family implications, all of which are important to consider in the therapeutic plan in our NICUs today.
Another related finding is the induction of lactase activity by feedings. Lactase, the enzyme responsible for the digestion of lactose, is present in the fetal intestine early in gestation, showing the greatest increase during the third trimester. Premature babies are relatively lactase deficient at birth. In one study, lactase activity was induced in preterm babies (26 to 30 weeks of gestation) by the initiation of enteral feeds. Of greatest significance, the highest levels of enzyme activity were seen with the introduction of “early” feeds (4 days old) as opposed to “standard” feeds (15 days old) and in human milk-fed versus formula-fed babies (
53). There was also an inverse correlation between lactase activity at 28 days and the time to achieve full enteral feeds. It appears that the level of lactase activity may be a marker of intestinal maturity, with a direct relationship between human milk use and the progression of gut maturity.
There are a large number of “bioactive components” in human milk that are not present in formulas. They variously provide anti-inflammatory effects, provide protection from infectious agents, are hormones and growth factors that influence development, or are immune function modulators (
54,
55,
56). This is an active area of research. At least a few of these bioactive factors have activity suggesting that they may be involved in GI maturation, growth, and motility (
54). Epidermal growth factor (EGF) is a major growth-promoting cytokine that stimulates proliferation of intestinal mucosa and epithelium and strengthens the mucosal barrier to antigens (
54). In an animal model in 1993, EGF isolated from human milk was shown to facilitate gut healing after induced injury (
57). Since then, EGF has been shown to be critical to the maturation and healing of the intestinal mucosa. Resistant to low pH and digestive enzymes, it stimulates intestinal enterocytes to increase deoxyribonucleic acid (DNA) synthesis, cell division, absorbance of water and glucose, and protein synthesis. EGF inhibits programmed cell death and corrects alterations in intestinal and liver tight junction proteins induced by proinflammatory tumor necrosis factor (TNF)-α. Heparin-binding EGF is the primary growth factor responsible for damage resolution following hypoxia, ischemia-reperfusion injury, hemorrhagic shock/resuscitation injury, and necrotizing enterocolitis (NEC). The average EGF level in colostrum is 2,000-fold higher, and in mature milk is 100-fold higher than in maternal serum. Preterm milk contains higher levels of EGF than does term milk (
54). Other factors identified in human milk, known as human growth factors I, II, and III and insulin-like growth factor, have been shown to have growth-promoting functions, including stimulation of DNA synthesis and cellular proliferation.
In vivo studies in animal species have shown remarkable increases in the mass of intestinal mucosa after feedings with colostrum, which contains these factors, but not after feedings with artificial milk (
58,
59).
Host Defense Advantages
Another of the extraordinary advantages of the use of human milk over formula in the preterm and NICU patient is the effect on host defense and infections. This advantage alone is enough to make the use of human milk the standard in this population. The myriad of hormones, factors, cytokines, proteins, enzymes, nucleotides, antioxidants, immunoglobulins, and live, functioning cell types such as lymphocytes, macrophages, neutrophils, and natural killer cells, as well as probiotic bacteria contained within human milk (
54,
55,
56), their interactions, and the milieu in which they effect action, is not and cannot ever be duplicated in an artificial milk. The more we discover, the more we find we have yet to uncover.
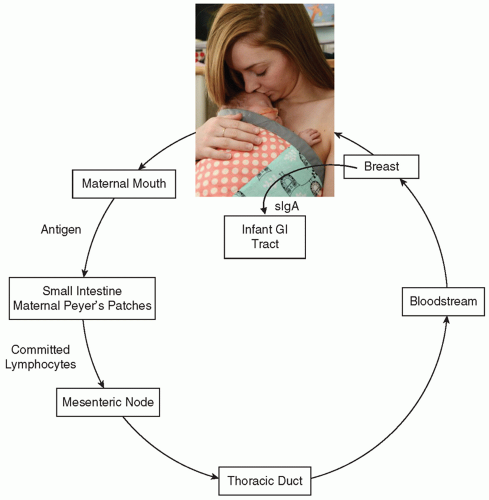
One can think of human milk not only as the perfect form of nutrition but also as “baby’s first immunization.” One type of immunization is the passive transfer of antibody. Examples include the use of intravenous immune globulin, tetanus immune globulin, or rabies immune globulin. The immunoglobulins represented in a mother’s milk are a history of many of the infectious agents she has been exposed to, and developed antibody to, during her lifetime. By transferring these antibodies to her baby through her milk, she is in effect “immunizing” her baby against those organisms. This process is a continuum from the one begun
in utero by the passage of maternal antibody across the placenta. Taking this one step further is the concept of the enteromammary immune system, first proposed in 1979 by Kleinman and Walker (
60) (
Fig. 21.2). In this schema, antigen presented to the maternal mouth and gut is brought into the proximity of lymphoid follicles in the maternal GI tract known as gut-associated lymphoid tissue (GALT). The antigen’s presence commits maternal lymphoblasts to specific IgA production against that antigen. These lymphoblasts migrate via the mesenteric nodes and the thoracic duct into the systemic circulation, in which they find their way into the active mammary tissue. There, these cells manufacture sIgA, which is secreted into the milk. When the infant ingests the milk, the immunoglobulin present functions in the infant’s gut as protection against a specific pathogen. Most sIgA is not absorbed in the infant’s intestine but rather plays an active role in mucosal defense. Although intact immunoglobulin has been found in infant urine, signifying some systemic absorption, most survives intact in the GI tract and is excreted into the feces. The environment of the NICU is full of potentially pathogenic organisms. What makes this concept of the enteromammary immune system even more appealing is that during skin-to-skin (kangaroo) care, a neonate is held by the mother at her chest and is touched, kissed, and caressed. The mother exposes herself to any potential pathogens to which her baby has also been in contact. Through the enteromammary immune system, she makes antibody to those particular organisms and then, in subsequent feedings, she passes that antibody to her baby. Imagine—individually prepared immunizations to help protect each infant in the NICU!
Similarly to our previous discussion of concentrations of specific nutrients, many of these immune modulators are in higher concentration in preterm milk than term milk, helping to compensate for the premature baby’s immature immune function (
Table 21.4). When major factors were quantified and compared between colostrum of mothers delivering both prematurely (28 to 36 weeks) and at term (38 to 40 weeks), mean concentrations of IgA, lysozyme, lactoferrin, and absolute counts of total cells, macrophages, lymphocytes, and neutrophils were found to be significantly higher in the preterm colostrum (
61). The degree of prematurity did not influence the anti-infective levels in the colostrum. However, the total cells and macrophages were significantly higher in the colostrum of mothers delivering at 28 to 32 weeks of gestation compared to 33 to 36 weeks of gestation (
p < 0.05) (
61). These differences make the use of early colostrum and human milk critical in the care of premature and sick babies, both as prevention and possibly in treatment of infectious disease.
Another factor in improved immune defense with the use of human milk is the affect on fecal flora. The normal flora of the intestines of a breastfed infant is predominantly the grampositive bacterium
Lactobacillus bifidus. Non-human milk-fed babies are colonized with many more types of bacteria, most of which are potentially pathogenic gram-negative bacteria. With establishment of
Lactobacillus as the predominant flora inhabiting
the premature infant’s GI tract, the likelihood of a serious or life-threatening gram-negative infection would be expected to decrease.
It has long been known that breastfed babies are at decreased risk of a number of infectious diseases—respiratory infections, otitis media, gastroenteritis, and diarrhea—and are at decreased risk of mortality. These protections accrue in a dose-dependent fashion —that is, the more human milk a baby receives, statistically the better protected they are. Although much of the earlier work was done in developing countries, there are now also many studies that show significant impact in populations in the developed world (
32,
33). As discussed previously, one study estimates 911 excess deaths per year in the United States due to lack of breastfeeding, nearly all of whom are infants (
34). It is probably fair to assume that babies admitted to NICUs, once attaining term corrected GA and discharged to home, will accrue similar advantages from breastfeeding/human milk as will their healthier term counterparts in these studies. But even more importantly for our population, an increasing body of research has accumulated examining the effects of a diet of human milk compared to preterm formula in premature and low-birth-weight babies, with respect to clinical infections. The data are clear—premature infants fed human milk are at significantly less risk of serious diseases, including NEC, urinary tract infections, sepsis, and meningitis.
As early as 1971, a report from Sweden showed a protective effect of breastfeeding against sepsis in the newborn (
62). Then, in 1980, Narayanan and her group in India reported that even partial use of human milk (
63) and subsequently exclusive use (
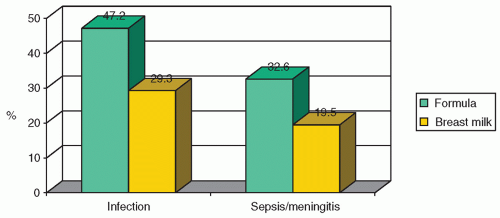
64) could significantly decrease the incidence of infection in a premature and low-birth-weight population. Infections noted were sepsis, diarrhea, pneumonia, meningitis, conjunctivitis, pyoderma, thrush, and upper respiratory infections. The strongest effect was noted in those babies exclusively fed human milk, followed by those fed partial feedings of human milk, thus indicating a dose-response effect. Hylander et al. examined the incidence of infection among VLBW infants, in relationship to their type of feeding, while controlling for confounding factors. They found that the incidence of infection (human milk 29.3% vs. formula 47.2%) and sepsis/meningitis (human milk 19.5% vs. formula 32.6%) differed significantly by type of feeding (
Fig. 21.3) (
65).
In 1999, Schanler et al. (
66) showed that in a group of premature infants between 26 to 30 weeks of gestation, those fed predominantly fortified human milk were discharged earlier (73 ± 19 vs. 88 ± 47 days) and had lower incidence of NEC and late-onset sepsis than did infants fed preterm formula. These data on NEC
confirm a previous study by Lucas and Cole (
67), who showed that in a cohort of 926 infants with birth weights below 1,850 g, formula-fed babies were 6 to 10 times more likely to develop NEC than were those fed human milk exclusively and three times more likely than were babies who received a combination of human milk and formula supplements, again demonstrating the dose-response effect of human milk. Recent studies show that using an exclusively human milk diet (human milk, either mother’s own or donor with human milk-derived fortifier if required) can reduce the incidence of NEC to virtually zero (
68,
69). Further study of the use of donor milk compared to mother’s own milk and exploration of issues pertaining to the high cost of the currently single manufacturer human milk-derived fortifier (which precludes its use by many NICUs) is required. While reviewing the data on NEC, it is also important to point out that, in addition to these significant clinical decreases in incidence, there are also concomitant significant economic savings. This is an enormous issue to practitioners, to the health care system as a whole, and certainly to the individual families we care for. A reduction in the cases of NEC would engender significant savings in medical charges and LOS. Bisquera et al.(
70) showed that infants with surgical NEC exceeded LOS by 60 days over matched controls and those with medical NEC by 22 days over controls. Based on LOS, the estimated total hospital charges per baby for surgical NEC averaged $186,200 more and for medical NEC $73,700 more than controls. This translated into yearly additional hospital charges at their institution for NEC of $6.5 million or $216,666 per survivor. A model using data from this study showed that, given these costs of NEC and the cost of providing human milk-derived fortifier, significant cost savings could be appreciated by treating all infants less than 1,250 g birth weight with exclusively human milk products (
71).
It appears clear that an exclusively human milk diet is protective in fragile preterm babies. In the previously discussed Hylander et al. study (
65), the human milk-fed group also received supplements of formula and still showed significant benefit. Lucas and Cole showed that looking at NEC, partial human milk feeding was protective but less so than exclusive human milk feedings (
67). Furman et al. (
72), looking at 119 VLBW babies, identified a daily threshold amount of at least 50 mL/kg/d of maternal milk through week 4 of life as needed to decrease rates of sepsis in this group. It has also been shown that upper respiratory symptoms are reduced for those low-birth-weight babies who continue to receive human milk after discharge from the NICU through 7 months corrected age and also decreased occurrence of rehospitalization in the first year of life (
73). Although not significant, a trend also appears to exist for otitis media, bronchiolitis, and gastroenteritis. More studies with larger cohorts are needed to confirm these data.
There is no longer any doubt that by providing human milk, we do make a difference in the incidence and impact of serious potential morbidities in even the tiniest of our patients in a dose-response manner. That being the case, with all the potential morbidities that our patients face, and the costs both economically and emotionally for these families and society, it is imperative upon us to make human milk the standard for nutrition in neonatal intensive care.
Neurodevelopmental Advantages
Improved cognitive development has been demonstrated in premature babies who receive human milk. In 1988, Morley and associates showed an 8-point cognitive advantage using the Bayley Scales of Infant Development for 771 infants with birth weights less than 1,850 g (
74). After controlling for demographic and perinatal factors, a 4.3-point advantage remained. When this cohort was followed up at 7.5 to 8 years of age, infants who had received human milk by tube (rather than breastfeeding) continued to show an 8.3-point IQ advantage (over half a standard deviation) even after adjustments were made for difference in mother’s education and social class (
75). They also showed a dose-response relationship between the proportion of human milk in the diet and subsequent IQ. There has been one meta-analysis of controlled studies looking at the question of human milk and cognitive development, which demonstrated a 3.16-point higher score for cognitive development in human milk-fed babies compared with formula-fed babies after adjustment for significant covariates (
76). This difference was observed as early as 6 months and was sustained through 15 years of age, the last time of reliable measurement. Longer duration of breastfeeding was accompanied by greater differences in cognitive development (dose-related response). Whereas normal weight infants showed a 2.66-point difference in IQ scores between breastfed and formula-fed groups, a 5.18-point difference was demonstrated in low-birth-weight infants. The results of this meta-analysis suggest that not only does human milk contribute to optimal neurodevelopment but the effect is even more striking in the higher-risk low-birth-weight infants.
Vohr et al. (
77,
78) followed a cohort of 1,035 babies who were less than 800 g at birth, enrolled in the National Institute of Child Health and Human Development Neonatal Research Network Glutamine Trial at 18 and then 30 months corrected age. Multivariate analyses, adjusting for confounders, confirmed a significant independent association of human milk on all four primary outcomes: the mean Bayley Mental Development Index, Psychomotor Development Index, Behavior Rating Scale, and incidence of rehospitalization. At 18 months, for every 10-mL/kg/d increase in human milk ingested, the Mental Development Index increased by 0.53 points, the Psychomotor Development Index increased by 0.63 points, the Behavior Rating Scale percentile score increased by 0.82 points, and the likelihood of rehospitalization decreased by 6%. In an effort to identify a threshold effect of human milk, the mean volume of human milk per kilogram per day during the hospitalization was calculated, and infants in the human milk group were divided into quintiles of human milk ingested adjusted for confounders. As every 10 mL/kg/d human milk contributed 0.53 points to the Bayley Mental Development Index, the authors suggest that the impact of human milk ingestion
during the hospitalization for infants in the highest quintile (110 mL/kg/d) on the Bayley Mental Development Index would be 10 × 0.53 or 5.3 points. They postulate that an increase of 5 IQ points in this population (one-third of a standard deviation) could be significant enough to impact need for early intervention and special education services (
77). At 30 months corrected age, the benefits remained. For every 10 mL/kg/d increase in human milk, the Mental Developmental Index increased by 0.59 points, the Psychomotor Developmental Index by 0.56 points, the total behavior percentile score by 0.99 points, and the risk of rehospitalization between discharge and 30 months decreased by 5% (
78).
Postulated to be responsible for at least a portion of these neurodevelopmental advantages of human milk is the presence of LC-PUFAs, which until recently, were not present in formulas (see also
Chapter 20 for a more in-depth review). DHA normally accounts for greater than one-third of the total fatty acids of the gray matter of the brain and the retina of the eye (
79). Most of the prenatal accumulation of DHA in these tissues occurs in the third trimester —thus by definition, premature infants are deficient compared to their term counterparts. Animal studies have shown that deficiency of DHA in neural tissues during development leads to behavioral and retinal changes.
Other examples of the effects of human milk on neurologic maturation have also been observed. Premature infants receiving human milk have been shown to have faster brainstem maturation than do those receiving formula (
80). Visual acuity and the development of retinopathy of prematurity (ROP) have also been studied. A number of studies were performed before the routine addition of the LC-PUFAs to preterm formulas. In one, improved retinal function was present in LC-PUFA sufficient VLBW neonates fed human milk or supplemented formula, as compared to a group receiving unsupplemented formula (
81). Better visual evoked potentials and acuity in both preterm and term infants at 57 weeks postconception have been seen in those fed human milk as opposed to those fed formula (
82). Additionally, another interesting study in which healthy term infants who breastfed to 4 or 6 months, and then were weaned, were randomly assigned to commercial formulas with or without DHA and arachidonic acid (AA) supplements. At 1 year of age, the level of DHA as measured in the red blood cells was reduced by 50% from weaning level in the unsupplemented group, although there was an increase of 24% in the supplemented group (
83). The conclusions drawn from this study were that the critical period during which a dietary supply of DHA and AA can contribute to optimizing visual development in term infants extends through the first year of life. This supports the AAP recommendation that breastfeeding continue through the first year of life (
1), and begs consideration then of the length of time breastfeeding should be encouraged and supported for the baby born prematurely. The incidence of ROP in relation to human milk feedings has been investigated. A secondary analysis of data collected during two multicenter randomized controlled trials (RCTs) in Italy revealed that ROP incidence (at any stage) was significantly lower in infants fed maternal milk as compared to formula-fed neonates; the same difference in incidence held for threshold ROP (
84). Another recent study looking at short-term outcomes in preterm infants detected lower rates of ROP in a subgroup of breastfed infants born at 24 to 28 weeks’ GA; the difference did not reach statistical significance using univariate analysis (
p = 0.06). However, using multivariate analysis, the incidence of ROP stage III among this subgroup was significantly lower (
p = 0.022) (
85). The etiology of ROP is clearly complicated and is not based simply upon the nature of the enteral feedings provided. The components in human milk that have the protective effects, be they the LC-PUFAs or bioactive factors, require further study.
Physiologic Advantages
Breastfeeding is widely assumed to be more stressful than is bottle-feeding for premature infants; an assumption that continues to lead, in many intensive care units, to the introduction of bottle-feedings as the first oral feeds, and postponing attempts at breastfeeding until babies “can prove themselves with the bottle.” There are also often concerns for the small premature baby’s ability to maintain temperature while breastfeeding, leading to “rules” restricting initiating breastfeeding until a certain weight is achieved. Additionally, there is the widely held belief that the “suck-swallow-breathe” mechanism is not mature until approximately 34 weeks of gestation. The fear that premature babies will choke, desaturate, and aspirate leads to more “rules” dictating that oral feeds, including breastfeeding, not be initiated until at least 34 weeks of corrected GA. It is important to point out that there is no scientific evidence to back any of these statements. On the contrary, the opposite is the case as we will discuss. It is also important to understand that these policies, in addition to being unsupported scientifically, are also harmful in that they (a) preclude premature babies and their mothers from early breastfeeding experiences; (b) allow babies whose mothers want to breastfeed to learn to suck from a bottle, which then in many cases makes it difficult for them to transfer to the breast; and (c) introduce breastfeeding so late in the hospital stay that, in addition to struggling to overcome what they have learned with an artificial nipple, the mother and baby are often discharged before they have had time to learn together and develop the confidence and skills necessary to allow successful breastfeeding, especially with the help they can receive from the NICU lactation staff. It is important to reemphasize our medical dictum in this circumstance: Primum non nocere.
Data do exist that enable us to develop breastfeeding policies that are physiologic, safe, and supportive of breastfeeding. As early as the 1980s, Meier was publishing data concerning physiologic stability at breastfeeding compared to bottle-feeding. She was able to show that in babies less than 1,500 g at the time of first feeding, different sucking mechanisms were employed for breast and bottle, with better coordination of suck-swallow-breathe during breastfeeding, particularly in the smaller, less mature babies. Concomitantly, there are markedly different patterns of transcutaneous oxygen pressure (tcPO
2) recorded for the two methods of feeding. TcPO
2 patterns suggest less ventilatory interruption during breastfeeding than during bottle-feeding, with greater declines in tcPO
2 recorded during bottle-feeding than during breastfeeding over the course of a feeding session. Additionally, infants stay significantly warmer during breastfeeding than during bottle-feeding (
86,
87,
88). Similar studies done by Bier and colleagues in first VLBW (
89) and then extremely low-birth-weight (ELBW) (
90) infants showed that they could tolerate beginning both breast- and bottle-feedings at the same PNA, that they were less likely to have oxygen desaturations to less than 90% during breastfeeding, and that they had lower intakes during breastfeeding. The lower intakes have been observed in a number of studies. It is postulated that this is due to several factors: better paced control of suck-swallow-breathe during breastfeeding than during bottle-feeding where the artificial nipple releases fluid regardless of whether the infant is ready to accept a bolus or not, possible lower milk volumes in some of the breastfeeding mothers, and weaker sucks in some of the infants resulting in less milk released with breastfeeding. These may all be factors in the relatively safer physiologic state when breastfeeding than bottle-feeding. Given that there are no data supporting the safety of initiating feeding with artificial nipples, and that there are data to show that during breastfeeding premature babies are more physiologically stable, it is therefore reasonable, safe, and scientifically sound for mothers to place their babies nuzzling at breast and beginning early steps toward breastfeeding, once they are physiologically ready, and there is no basis for requiring bottle-feeding as first feeds, if mothers intend to breastfeed.