Bladder/Cloacal Exstrophy, and Prune Belly Syndrome
John P. Gearhart
Dominic Frimberger
Department of Urology, Johns Hopkins Children’s Center, Baltimore, Maryland 21287.
Department of Urology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287.
BLADDER EXSTROPHY
Incidence
The incidence of bladder exstrophy has been estimated between 1 in 10,000 and 1 in 50,000 live births (1,2). However, data from the International Clearinghouse for Birth Defects monitoring system estimated the incidence to be 3.3 per 100,000 live births (3). A 5:1 to 6:1 ratio of male-to-female rate exists (1).
Embryology
Mesodermal ingrowth between the ectoderm and endodermal layers of the bilaminar cloacal membrane results in formation of the lower abdominal musculature and pelvic bones. After mesenchymal ingrowth occurs, downward growth of the rectal septum divides the cloaca into a bladder anteriorly and a rectum posteriorly. The genital tubercles migrate medially and fuse in the midline cephalad to the dorsal membrane before it perforates. The cloacal membrane is subject to premature rupture, depending on the extent of the infraumbilical defect. The stage of development when the membrane rupture occurs determines whether bladder exstrophy, cloacal exstrophy, or epispadias results (4).
The most likely theory of embryonic development in exstrophy describes the basic defect as an abnormal lower overdevelopment of the cloacal membrane, preventing medial migration of the mesenchymal tissue, and therefore, proper abdominal wall development (5). The timing of the rupture of this cloacal defect determines the severity of the disorder. The highest incidence with 60% is central perforations resulting in classic exstrophy, where 30% have exstrophy variants and 10% cloacal exstrophy.
Other theories have been offered concerning the cause of the exstrophy-epispadias complex. These include (1) abnormal development of the genital hillocks, with fusion in the midline below rather than above the cloacal membrane; (2) abnormal caudal insertion of the body stalk, with failure of the interposition of the mesenchymal tissue in the midline so translocation of the cloaca into the depths of the abdominal cavity does not occur; or (3) a cloacal membrane that remains in a superficial infraumbilical position, and remains in an unstable embryonic state with a strong tendency to disintegrate (4,6,7).
Predisposing Factors
Some evidence exists for genetic predisposition of exstrophy and epispadias. The risk for recurrence of bladder exstrophy and epispadias in any given family is 1 in 275 births (8). The likelihood of an exstrophic parent producing a child with exstrophy is about 1 in 70, or 500 times the risk of the general population. More recent data have shown a sevenfold increase in exstrophy and cloacal exstrophy associated with the use of assisted reproductive technology such as introcystoplasmic sperm injection (9).
Prenatal Diagnosis
Exstrophy of the bladder is rarely diagnosed by prenatal ultrasound. The main criteria for the prenatal diagnosis of exstrophy includes absence of bladder filling; lower abdominal mass, which becomes more protuberant as the pregnancy proceeds; a low-set umbilicus; normal scrotum; separation of the pubic rami; and difficulties determining the sex of the baby. In addition to these data, bladder exstrophy should always be suspected on the basis of nonvisualization of the bladder and a low-set umbilicus.
Anatomic Malformations and Histologic Differences in the Exstrophy Complex
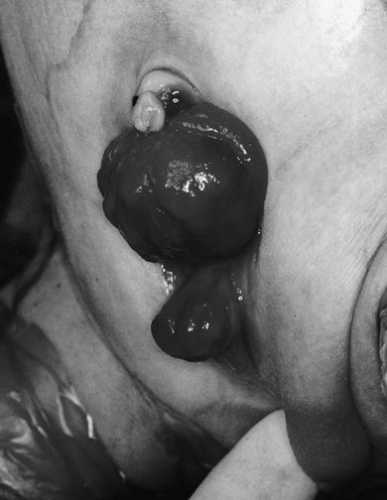
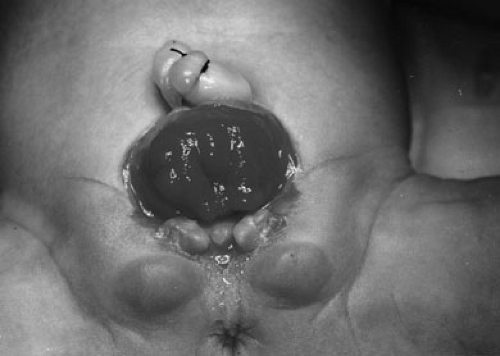
The diagnosis is made at birth. The bladder plate characteristically protrudes just beneath the umbilical cord with divergent rectus muscles on either side leading to separated pubic bones (Fig. 70-1). This separation is caused by outward rotation of the innominate bones, eversion of the pubic rami, and a 30% shortage of bone in the pubic ramus. In boys, there is a short phallus due to 50% deficiency of corporal length (10) with a dorsourethral plate, splayed glans, and dorsal chordee. The scrotum is generally normally developed, although the testes are often located in the distal inguinal canal. In girls, the mons, clitoris, and labia are separated, and the vaginal orifice is displaced anteriorly (Fig. 70-2).
Bilateral inguinal hernias are common due to a lack of obliquity of the inguinal canal combined with large internal/external rings. Inguinal hernia is reported in 82% of males and 11% of females (11).
Three-dimensional computed tomography (CT) demonstrates the anterior segment of the levator ani to be shorter and the posterior segment of the levator ani longer in exstrophy patients compared with normal controls. In addition, the transverse diameter of the levator hiatus is two times larger and the length 1.3 times greater, respectively. These anatomic findings cause a more anterior/superior rotation of the pelvic floor in exstrophy patients vs. normal controls. Although the levator muscle group in normal controls is evenly distributed anteriorly and posteriorly to the rectum, there is an uneven 70% to 30% posterior to anterior distribution in the exstrophic pelvis. Subsequently, the anus is anteriorly placed and sometimes patulous as part of the posterior extent of the myofascial defect (12,13). These musculoskeletal malformations explain the increased rate of rectal prolapse especially in the female exstrophy population (1).
The collagen/smooth muscle ratio was found to be 0.833 ± 0.46 instead of the 0.33 ± 0.11 ratio in normal control children, but the ratio normalized after successful closure (14). Although there is a decrease in the neural innervation of the bladder exstrophy muscle at the time of birth, the bladder musculature contains a normal compliment of muscarinic receptors for contractile stimulation staining (15). Using neuropeptides, evidence of abnormal innervation was not found in the classic bladder extrophy, but was found in the cloacal exstrophy children. Bladder specimens from newborns with classic bladder exstrophy were stained antibodies to the S 100 protein. Although the large nerve fibers were preserved, there was a significant reduction in myelinated and smaller nerve fibers (15). Using electron microscopy, significant differences in the ultrastructural nature of the intracellular organelles appear even more severe after failed initial bladder closure. Although these ultrastructural abnormalities are also seen if the bladder fails to grow, the group that obtained an adequate capacity after initial closure for bladder neck repair had only minimal changes of the bladder smooth muscle cells (16).
CT scans of patients with bladder exstrophy have shown that the posterior and anterior pelvic segments are externally rotated by a mean of 12 and 18 degrees on each side, respectively, with significant acetabular retroversion of the
anterior pelvic segment. No difference was found either in the length of the posterior segment or in the width of the sacrum (17).
anterior pelvic segment. No difference was found either in the length of the posterior segment or in the width of the sacrum (17).
Assessment and Management in the Neonatal Period
It is essential that the newborn with exstrophy be seen by a pediatric genitourinary surgeon with a special interest and experience in this problem because the impact of a major birth defect is significantly worsened by inappropriate initial management.
The bladder mucosa is easily injured and inflamed; therefore, the umbilical cord should be ligated with a strong silk suture, rather than clamped to avoid mucosal abrasion. The bladder is best protected with a clear plastic wrap and should not be covered with moistened or petroleum-coated gauze. Each time the diaper is changed, the bladder surface is irrigated with sterile saline and the plastic wrap replaced.
General pediatric and cardiopulmonary assessment should be completed with an eye toward the likelihood of major surgery in the first 48 hours of life. An ultrasound of the kidneys is performed to rule out hydronephrosis and a radionucleotide scan added if abnormalities are seen.
The parents have to be reassured that children with classic bladder exstrophy are in general healthy, robust infants with the prospect of a very normal life (1). Effective reconstruction to allow urinary storage, drainage, and control can be expected with an acceptable cosmetic appearance. The support of psychologists, nurses, and parents of other children with exstrophy is invaluable and is available at a large medical center with an interest in this problem. There are also multiple international support groups, the most prominent being the Association for the Bladder Exstrophy Community.
Role and Technique of Osteotomy
Closure of the pelvic ring is important to the eventual attainment of urinary continence and appropriate cosmesis of the abdominal wall. If the operation can be carried out within the first 72 hours of life, the pelvic ring can sometimes be closed effectively without the need for osteotomy (1). When the pubic diastasis is greater than 4 cm or in the condition of a failed prior closure, osteotomy is essential to the closure of the pelvic ring and should be performed at the same time as the bladder closure (18).
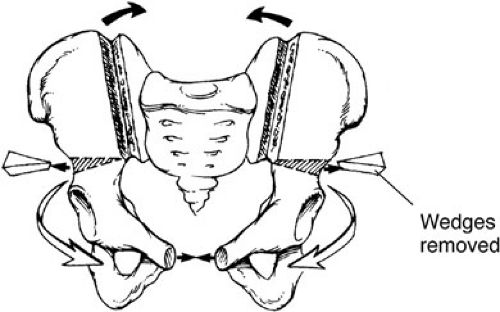
The most widespread pelvic osteotomy used in the world today is the transverse innominate and vertical iliac osteotomy performed from an anterior approach dividing the innominate bone above the acetabulum (17,18) (Fig. 70-3). An incision is made at the junction of the trunk and the legs, both sides of the innominate bone are exposed simultaneously, and horizontal osteotomies are performed using a Gigli saw. The osteotomy extends from 5 mm above the anterior inferior iliac spine to the most cranial part of the sciatic notch. Bilateral iliac osteotomies are performed at the same time using an osteotome and leaving the posterior table intact, giving the pelvis a better contour and mobility. The infant is already in supine position and prepared so the bladder closure can begin immediately. Pins for the external fixating device are inserted after bladder, but before the wound closure. This approach provides improved symphyseal approximation, making the midline closure easier and decreasing the likelihood of dehiscence. Postoperatively, the external fixator is left in place for 4 to 6 weeks, whereas Buck’s traction is maintained with the legs supported only on a pillow. Newborns undergoing exstrophy closure without osteotomy are maintained in modified Bryant’s traction for 4 weeks. The hips are kept at 90 degrees flexion with the knees straight.
Bladder and Posterior Urethra Closure in the Modern Repair
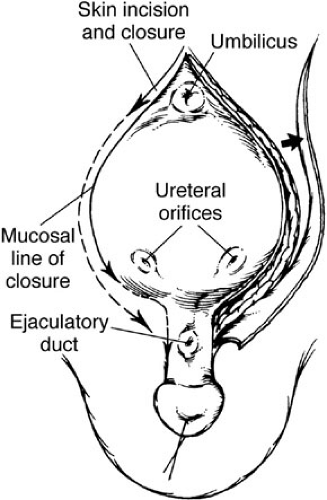
A strip of mucosa 2 cm wide extending from the distal trigone to below the verumontanum and out onto the penis in boys and to the vaginal orifice in girls outlines the prostatic and posterior urethral construction (Fig. 70-4). The male urethral groove is usually of adequate length to allow for primary closure. This is necessary, especially when the prostate is up near the base of the glans. The groove can be elongated by using paraexstrophy skin flaps (19). When these flaps are needed, great care should be exercised to follow plastic surgical principles of rotational skin flaps. An incision is made outlining the bladder mucosa and the prostatic plate. The urethral groove is transected distal to the verumontanum. In the staged approach, it is no longer necessary to free the corpora extensively or dissect
them completely in the midline because of the preference for later epispadias repair, as described in the modified Cantwell-Ransley approach. The urethra in the female patient does not require lengthening at the time of initial bladder closure, but can still be very short. By cutting the hymenal ring at 3 o’clock and 9 o’clock, an additional 0.5 cm or so of urethral length can be obtained.
them completely in the midline because of the preference for later epispadias repair, as described in the modified Cantwell-Ransley approach. The urethra in the female patient does not require lengthening at the time of initial bladder closure, but can still be very short. By cutting the hymenal ring at 3 o’clock and 9 o’clock, an additional 0.5 cm or so of urethral length can be obtained.
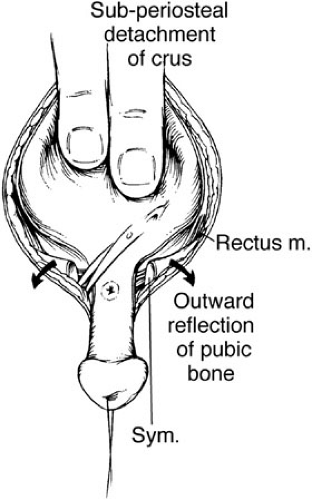
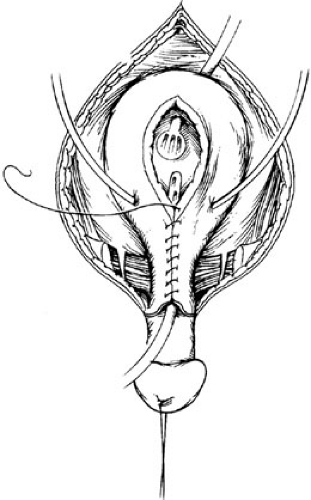
The umbilical area is dissected free and discarded. The bladder muscle is freed from the rectus sheath on each side. The peritoneum is exposed above the bladder, and a careful extraperitoneal dissection reveals the retropubic space on each side. The peritoneum is taken down from the dome of the bladder to allow the bladder to be sunken deep into the pelvis at the time of closure. Dissection is carried distally along the border between the bladder and the rectus down to the level of the urogenital diaphragm fibers. These fibers are incised sharply, and the diaphragm is detached subperiostally from the pubis bilaterally. This dissection must be carried onto the inferior ramus, and taken laterally and caudally down to the level of the levator hiatus to allow the bladder neck and posterior urethra the mobility to fall deeply within the pelvic ring (Fig. 70-5). A double-pronged skin hook can be inserted into the pelvic bone and pulled laterally to accentuate the urogenital diaphragm fibers. If this maneuver is not performed adequately, the vesicourethral unit will be brought anteriorly with pelvic closure in an unsatisfactory position for later reconstruction. The mucosa and muscle of the bladder are closed in the midline (Fig. 70-6). The urethra is closed proximally and out onto the penis so it can easily accommodate a 12 to 14 sound. This allows enough resistance to stimulate bladder growth and prevent bladder prolapse, but not too much to cause upper tract changes. The posterior urethra and bladder neck are buttressed to a second layer of local tissue, if possible. The bladder is drained by a suprapubic Malecot catheter for 4 weeks. The urethra is not stented. Ureteral stents provide urinary drainage for the first 3 weeks after surgery to avoid ureteral obstruction and hydronephrosis. The pelvis is closed by placing pressure on both greater trochanters, where horizontal mattress sutures using no. 2 nylon are placed in the pubis with the knot directed away from the urethra. A second stitch is placed caudal to the insertion of the rectus fascia, if possible, for added support. A simple umbilicoplasty is performed with a V-shaped flap, and the drainage tubes are brought out through this site.
In females, the mons and external genitalia are reconstructed at the time of initial exstrophy closure. The bifid clitoris is denuded medially and brought together in the
midline, along with labia minora reconstruction, creating a fourchette.
midline, along with labia minora reconstruction, creating a fourchette.
Postoperative Care After Initial Closure
Broad-spectrum antibiotics are administered before and during the procedure to convert a contaminated area into a clean surgical wound. These antibiotics are continued postoperatively and then converted to low-dose prophylactic antibiotics. The factors in achieving successful primary closure have been well documented. Urethral catheters, abdominal distension, infection, and poor nutrition appear to be associated with bladder prolapse and dehiscence (20). In addition, a review of a large series of failed exstrophy patients referred to Johns Hopkins stressed the importance of osteotomy, avoidance of urethral tubes, use of postoperative antibiotics, pelvic immobilization, ureteral stenting catheters, and maintaining patient comfort, as well as decreasing the amount of postoperative movement (21). The modern application of continuous caudal catheter anesthesia has been a great asset to the anesthesia techniques in these patients. After placing the catheter in a subcutaneous tunnel, it can be left in place for up to 2 weeks postoperatively and provides excellent continuous pain control.
Four weeks after closure, the residual urine is estimated by clamping the suprapubic tube and a urine culture is obtained before the child leaves the hospital. An ultrasound is performed to assess the status of the upper tracts and repeated regularly. The bladder outlet and urethra are calibrated with a sound or catheter and, if adequate and residual urines are low, the suprapubic tube is removed. Because primary closure creates a complete epispadias with incontinence, it is unusual to have much of a continent interval after the operation. Consequently, urethral calibration and cystoscopy, as well as renal ultrasound, should not only be performed for urinary retention, but also for prolonged continent periods. If uncontrollable hydronephrosis develops, revision of the bladder outlet must be considered. Rarely, a child requires urinary diversion because of upper tract deterioration, but if necessary it is usually performed using a nonrefluxing colon conduit to preserve renal function.
Epispadias Repair
Repair of the epispadias adds to the outlet resistance of the urinary stream and can subsequently contribute significantly to the development of a larger bladder capacity (22). Epispadias repair is carried out between 6 months and 1 year of age. To stimulate penile skin growth, preoperative testosterone is given intramuscularly. Regardless of the time of epispadias repair, the five goals include (1) achievement of potential penile length, (2) correction of dorsal chordee, (3) reconstruction of the penile urethra, (4) reconstruction of the glans penis, and (5) adequate skin coverage.
Penile lengthening has been described as a key component in initial bladder exstrophy closure and is best done at the time of initial exstrophy closure. Techniques for achieving penile length vary, but all have in common release of corporal tissue from the inferior pubic ramus, preservation of the neurovascular bundles, and achievement of maximal urethral length.
A penile disassembly technique where the urethral plate is totally taken from the corporal bodies, rolled into a tube, and then brought to the tip of the penis has been described. Initial reports of a small series of this technique have been favorable (23). However, in some patients, when the urethral plate is totally dissected from the glans it will not reach the tip of the penis and either a graft has to be used or the patient is made into a hypospadias condition, which will need to be repaired later (24,25). Considering the description of a 50% shortage in penile length between exstrophy patients and controls, it is the author’s opinion that any minimum of extra length obtained is not worth sacrificing the urethral plate and creating a hypospadias condition (10). The techniques of epispadias repair are described elsewhere in this text.
Bladder Neck Reconstruction
Timing is crucial to the success of bladder neck reconstruction. An adequate bladder capacity is an absolute prerequisite for success (26). The patient must have the desire to be dry and old enough to cooperate with toilet training. Under anesthesia, the bladder capacity is measured yearly after the child reaches 3 years of age. If the capacity is 85 mL or greater, bladder neck reconstruction can be considered. If the bladder does not achieve an adequate capacity after epispadias repair, injecting a bulking agent around the bladder neck, or ultimately, augmentation cystoplasty can be performed. Nearly all these children exhibit vesicoureteral reflux and antireflux procedure is required at the time of bladder neck repair (1).
Modified Young-Dees-Leadbetter Bladder Neck Reconstruction
The bladder is opened using a lower transverse incision. This is extended vertically in the midline, and the cervical closure of this incision narrows the area of the bladder neck at the end of the procedure. Reimplantation of ureters can be done in a transtrigonal or cephalotrigonal fashion.
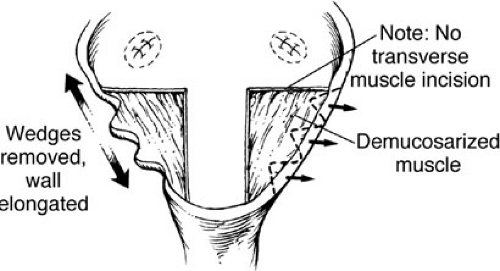
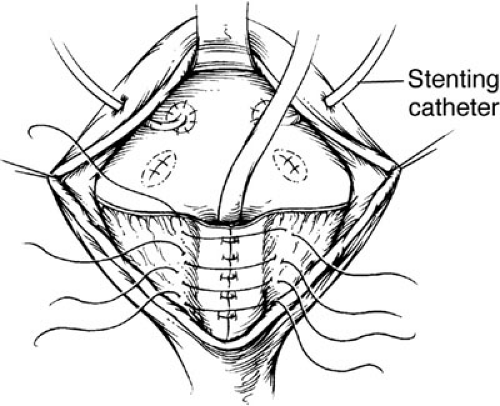
Bladder neck reconstruction is begun by outlining a posterior mucosal strip 15 to 18 mm wide by 3 cm in length, which extends from the midtrigone to the prostate and posterior urethra. A transverse, full thickness muscular incision is not performed because there is a significant risk of denervation and ischemia for the bladder neck. The bladder muscle lateral to the strip is denuded of mucosa. Multiple small incisions in the free edges of these triangles of muscle allow the reconstruction to assume a more cephalad position (Fig. 70-7). A mucosal strip is formed into a tube using interrupted sutures of 4-0 polyglycolic acid. Denuded mucosal flaps are overlapped, and sutures are placed with 3-0 polydioxanone sutures to reinforce the neobladder neck (Fig. 70-8). Reconstruction is performed over an 8F urethral catheter, which is removed at the end of the procedure. It is essential that the bladder neck be dissected completely free from the surrounding structures. This allows suspension of the bladder neck to the anterior fascia. Often, it is advantageous to split the symphyseal bar to enhance visualization. The bar is simply closed at the end of the procedure with heavy sutures of polydioxanone. Mobility should be restricted in the postoperative period to allow healing.
Postoperative Management After Continence and Reflux Procedures
The patients are kept on broad-spectrum antibiotics and intravenous (IV) fluids until taking food by mouth well. The ureteral stents are removed after 2 to 3 weeks. At 3 weeks, the suprapubic tube is clamped intermittently to initiate a voiding trial. Initially, the tube should not be clamped for more than 1 hour. Once the child is emptying the bladder satisfactorily, the suprapubic tube is removed. Frequent bladder and renal ultrasounds are obtained in the first few months after bladder neck reconstruction to ensure adequate emptying and to observe the status of the upper tracts. If voiding does not occur after clamping the suprapubic tube, a Foley catheter is placed under anesthesia. Sometimes cystoscopy and placement of a catheter over a guide wire is necessary to negotiate the urethral channel. The catheter is then left in place for 5 days, at which point another voiding trial is attempted. Children
failing repeated voiding trials are typically placed on intermittent catheterization. In some cases, these patients may have adequate storage capacity, but are unable to initiate bladder contractions sufficient for voiding. In other patients, bladder capacity may not increase necessitating bladder augmentation. If hydronephrosis is observed, the cause is likely ureterovesical junction obstruction or high voiding pressures. Urodynamics and a Lasix scan will aid in determining the correct diagnosis.
failing repeated voiding trials are typically placed on intermittent catheterization. In some cases, these patients may have adequate storage capacity, but are unable to initiate bladder contractions sufficient for voiding. In other patients, bladder capacity may not increase necessitating bladder augmentation. If hydronephrosis is observed, the cause is likely ureterovesical junction obstruction or high voiding pressures. Urodynamics and a Lasix scan will aid in determining the correct diagnosis.
Suppressive antibiotics are continued until complete bladder emptying is achieved. In some patients, recurrent infections will become a problem. After exclusion of high postvoid residuals, long-term antibiotic suppression is recommended. The bladder should be carefully monitored to ensure progress over time as the bladder function improves. Postoperative irritations may result in an extremely small bladder capacity at first. Patients are not familiar with the sensation of bladder filling or the need for detrusor contraction. Several months for adjustment are often required before a reasonable dry interval can be developed.
Combined Bladder Exstrophy and Epispadias Repair
Newborn exstrophy closure can be combined with epispadias repair. However, this approach requires good phallic length, a deep urethral groove, and an adequate amount of penile skin (24,25,27). This technique should only be attempted by an experienced exstrophy surgeon as the complications can be severe (28,29). One of the best applications of combined exstrophy and epispadias repair is in the patient undergoing delayed primary closure or reoperative exstrophy closure (29).
The combined closure of bladder exstrophy and epispadias repair is very similar to the beginning of the closure of bladder exstrophy and posterior urethra alone. Evaluation of the bladder template is performed by everting the bladder into the abdomen with a sterile gloved finger. This allows evaluation of the extent of the bladder plate, which may be larger than noted on visual inspection of the abdominal wall defect. The operative procedure begins and proceeds as with the standard closure of the bladder, posterior urethra, and abdominal wall with only a few differences. In reclosure, great care must be used to divide any remnants of the urogenital diaphragm fibers that were left behind after the failed initial closure. Once the urogenital diaphragm fibers have been incised, the entire vesicoureteral unit can be moved posteriorly into the pelvis. Placing the bladder neck and posterior urethra deep within the pelvic ring allows approximation of the levator and puborectalis sling, and provides an improved eventual continence rate.
After adequate dissection of the bladder and posterior urethra, attention is given to the dissection of the penis, corporal bodies, and urethral plate. This part of the procedure progresses much as in a standard epispadias repair and exstrophy closure with a few exceptions. The corporal bodies are not brought over the urethra until the pelvic bones are sewn together in the midline, thus rotating the corpora more medially and allowing less tension on the corpora in the midline. A small oblique drain is placed next to the bladder closure, and abdominal wall closure is completed. An 8F Firlit stent is used to stent the urethra for 2 weeks. The external fixating device is then attached to intrafragmentary pins and tightened. External fixation of the pelvis is maintained for 4 weeks in children undergoing primary closure and 6 to 8 weeks for those undergoing reclosure of the bladder. Follow-up is very much the same as in standard exstrophy closure with monitoring residual urine and upper tract imaging by ultrasonography before suprapubic tube removal. Although some patients have achieved long-term continence after the procedure, most ultimately require bladder neck reconstruction to become dry.
Overall Results After Bladder Exstrophy Repair
The overall outcome after bladder exstrophy repair has improved remarkably since the mid-1970s. The standard treatment of exstrophy in the United States has evolved from urinary diversion to functional reconstruction in almost all cases. Favorable overall long-term outcome depends on the success of all phases of surgical reconstruction. The importance of a successful initial closure cannot be overemphasized and should be performed in centers with a large experience. Failure of the initial closure markedly decreases the attainment of successful continence and, in most cases, ultimately required bladder augmentation (30). Continence is correctly defined as being dry for more than 3 hours. Socially continent patients achieve that goal during the day, but have bed-wetting incidences during nighttime. After modern repair, continence rates can be expected to be as high as 75% to 80% with preservation of renal function as documented in several large series (26,31,32,33). Appropriate patient selection, meticulous surgical technique and careful postoperative care are essential to obtaining consistent outcomes. The functional and cosmetic outcomes of the genital reconstruction are of high quality. Patients have a very low rate of urethrocutaneous fistula with a very acceptable cosmetic appearance (34). Most postpubertal patients were able to participate in satisfactory intercourse (35).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree