Approach to Fractures with Neurovascular Compromise
Scott H. Freedman
Brent R. King
Introduction
Neurovascular compromise should be considered whenever a child presents with an injured extremity. Although children with cool, pale, or cyanotic injured extremities are of obvious concern, serious injuries also can be subtle (1,2). It is essential that individuals who care for children in either the prehospital setting or the emergency department (ED) be adept in the evaluation and initial management of the injured extremity. Depending on the circumstances, such as a lengthy prehospital extrication or transport, it may be several hours before the child receives definitive orthopaedic care. Emergency personnel therefore may be expected to perform limb-saving procedures, including fracture reduction or limb manipulation in a neurovascularly impaired extremity. When the clinician is confronting the rare but serious entity of compartment syndrome, expedient diagnosis and management are essential. The emergency physician must be able to measure intracompartmental pressures if an orthopaedic surgeon is not available to do so.
This chapter highlights specific orthopaedic injuries in children that may be limb threatening (3,4,5,6,7). All pediatric age groups may be affected, but due to the nature of certain injury patterns, school-age children and adolescents are the usual victims. Specific information about splinting (Chapter 101), casting (Chapter 102), and exsanguinating hemorrhage (Chapter 28) is provided elsewhere in this text and will not be discussed in detail in this chapter.
Anatomy and Physiology
A comprehensive review of the musculoskeletal anatomy of each neurovascular structure of interest is beyond the scope of this chapter. The reader is referred to a basic clinical anatomy textbook or atlas should more detail be desired (8,9).
Upper Extremity
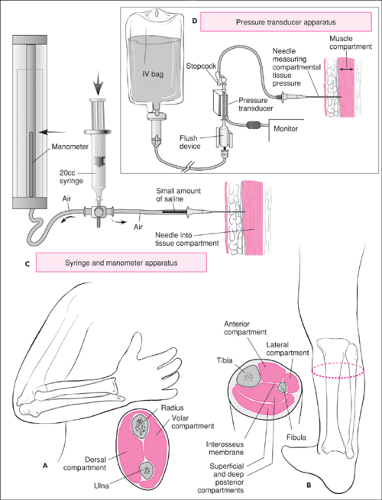
The forearm is divided into an anterior (volar) and posterior (dorsal) compartment by a fascial sheath, interosseus membrane, and intermuscular septum. Each compartment contains its own muscle groups, innervation, and blood supply. An injury to the forearm that causes an elevation in compartmental pressures can result in the development of compartment syndrome. A detailed discussion of compartment syndrome (8,9) is provided later in this chapter. Table 106.1 lists the anatomic structures and their functions in the forearm and leg compartments.
The elbow joint is a complex synovial hinge joint between the distal humerus and the proximal radius and ulna. The spool-shaped trochlea and the rounded capitulum of the humerus articulate with the trochlear notch of the ulna and the cupped radial head, respectively. In evaluating children, one must be knowledgeable of the age at which the ossification centers first appear and fuse so as not to confuse an ossification center with a fracture. This is particularly challenging when interpreting radiographs of the elbow. “CRITOE” is a commonly used mnemonic that can help the clinician remember the ossification centers of the elbow and the ages at which they appear and fuse (Table 106.2). In girls, the centers of ossification fuse at age 14 to 15 years, whereas in boys fusion occurs at around 18 to 21 years of age.
An additional important feature of the bones of the elbow has to do with the actual structure of the supracondylar region of the humerus. In children, the distal metaphysis of the humerus is flared and flat. Posteriorly it is indented due
to the olecranon fossa, and anteriorly it is thinned due to the coronoid fossa. The supracondylar region is a relatively weak section of bone that is somewhat susceptible to fracturing, especially when the ossification centers are not fused. The brachial artery and its branches, the radial and ulnar arteries, and the median and radial nerves are the principal neurovascular structures that could be damaged in a complex supracondylar elbow fracture.
to the olecranon fossa, and anteriorly it is thinned due to the coronoid fossa. The supracondylar region is a relatively weak section of bone that is somewhat susceptible to fracturing, especially when the ossification centers are not fused. The brachial artery and its branches, the radial and ulnar arteries, and the median and radial nerves are the principal neurovascular structures that could be damaged in a complex supracondylar elbow fracture.
TABLE 106.1 Compartment Structures of the Forearm and Leg | |
|---|---|
|
TABLE 106.2 Ossification Centers of the Elbow | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Lower Extremity
The lower limb consists of four parts: the pelvis, consisting of the hip bones (fusion of the ilium, ischium, and pubis) and their connections between the vertebral column and the femur; the thigh, containing the femur connecting the knee and hip; the lower leg, containing the tibia and fibula connecting the ankle and the knee; and the foot and the connection to the ankle. Because the greatest risk for neurovascular injuries in the lower extremity involves the knee and the compartments of the leg, much of the discussion will focus on these areas.
Knee
As with the elbow, the knee is a synovial hinge joint. Articulation at the knee is between the large rounded condyles of the distal femur, the flattened condyles of the proximal tibia, and the facets of the patella. Principal movement of the knee is flexion and extension, but some lateral and medial rotation is also possible.
Important neurovascular structures that traverse from the thigh to the calf lie deep within the popliteal fossa and are generally well protected from damage by a strong, thick fascial covering. However, with knee dislocations, displaced fractures through the physes of the proximal tibia or distal femur, or penetrating injuries to the fossa, there is a relatively high risk of concomitant neurovascular injuries to the leg and foot. The popliteal artery and vein, the small saphenous vein, the tibial and common peroneal nerves, and the end branch of the posterior femoral cutaneous nerve all are contained in the fossa.
If the popliteal artery is damaged (severed or partially lacerated or undergoing vasospasm), circulation to the leg could be compromised, with resultant ischemic damage. The primary structures that innervate the leg and foot pass through the popliteal fossa. The tibial nerve supplies articular branches to the knee and motor branches to the muscles of the posterior leg and the plantar flexors of the foot. It joins a branch of the common peroneal nerve to form the sural nerve, which innervates the lateral aspect of the ankle and sole of the foot. The common peroneal nerve supplies articular branches to the knee and proximal tibiofibular joints, motor branches to the dorsiflexor and evertor muscles of the foot, and cutaneous sensory branches that innervate the skin of the calf.
Lower Leg
A deep sheath of fascia encapsulates the leg by surrounding its contents and attaching to the medial and lateral borders of the tibia. Two intermuscular septa together with an interosseous membrane between the tibia and fibula divide the leg into three compartments: anterior, posterior, and lateral (Fig. 106.1B). The posterior compartment is further subdivided into a superficial and deep compartment by a transverse intermuscular
septum passing between the tibia and fibula. As with the forearm, each compartment contains its own muscle group, blood supply, and nerve supply. Because the structures of the leg are well contained within these thick fascial septae, an injury that results in increased swelling may lead to increased compartmental pressures. This compartmentalization, along with the high incidence of injuries to the leg, make it the most common site for the development of compartment syndrome. It is therefore important to know the structures contained in the leg compartments (Table 106.1).
septum passing between the tibia and fibula. As with the forearm, each compartment contains its own muscle group, blood supply, and nerve supply. Because the structures of the leg are well contained within these thick fascial septae, an injury that results in increased swelling may lead to increased compartmental pressures. This compartmentalization, along with the high incidence of injuries to the leg, make it the most common site for the development of compartment syndrome. It is therefore important to know the structures contained in the leg compartments (Table 106.1).
Neurovascular Examination of The Injured Extremity
Before obtaining radiographs and administering anesthesia, a thorough physical examination of an injured limb should be performed and documented. Examination of an injured child is often emotionally charged and difficult from the onset. Every effort must be made to establish rapport with and comfort the child. The examination should never be rushed. It may be helpful to have a family member present who will serve as a calming influence for the fearful or uncooperative child. Every effort should be made to perform a complete assessment without causing trauma or excessive additional pain.
Initial appropriate immobilization of the injured extremity is essential. A simple padded splint should be applied to the suspected fracture, with care taken to include the joints above and below the injury. At first, it is best to splint the injured limb in its deformed position to provide stability and comfort while minimizing further soft-tissue or neurovascular damage. The splinted extremity should then be elevated and ice applied in a manner that will not interfere with the remainder of the examination (see Chapter 101).
In some cases, the patient’s injury is so painful that an adequate examination is impossible. When this occurs, analgesia should not be withheld and in fact will likely improve the examination by localizing findings. It is recommended that an intravenous narcotic be titrated (e.g., morphine sulfate 0.05 to 0.2 mg/kg or fentanyl 1 to 3 μg/kg) until adequate analgesia is obtained. The goal is to decrease pain and fear without inducing a state of deep sedation (see Chapter 33). It is obviously necessary to exclude potentially life-threatening injuries (e.g., injuries to the head, chest, or abdomen) prior to performing a detailed examination of an extremity injury.
The injury should always be carefully inspected before palpation or movement. Inspection is begun by first removing all clothing and jewelry that may interfere with complete visualization of both the injured limb and the contralateral limb. Dressings may need to be temporarily removed. If the injured extremity has been immobilized, it is still imperative to perform a complete neurovascular examination. The splint or traction device may have been inadequately applied, and a long bone fracture may be improperly aligned, shortened, or excessively stretched, with resulting neurovascular compromise. The appliance must be properly adjusted and the extremity fully reassessed.
The patient should initially be observed undisturbed at rest. A child will always attempt to maintain the injured limb in a position of maximal comfort. The clinician should observe for active movement of the fingers and toes distal to the injury and compare findings to the contralateral limb. Any gross bony or musculotendon deformities, swelling, and overlying ecchymoses should also be noted. Lacerations, punctures, gunshot wounds (probable entry and exit sites), and degloving injuries may indicate an open fracture. Active bleeding, arterial or venous in nature, should be noted and controlled by applying direct pressure (see Chapter 28). The amount of blood loss from an open fracture is often grossly underestimated (1). Because major nerves nearly always run together with arteries, blind clamping of bleeding arteries should be avoided, as this may cause injury to the accompanying nerve. Furthermore, applying a tight tourniquet proximal to the bleeding vessel may lead to irreversible ischemic damage. This method of hemorrhage control should be reserved for an irreversibly damaged or crushed extremity for which the application of direct pressure is ineffective (1).
Circulation is assessed next. Pallor or cyanosis is noted distal to the injured part, and the color is compared with that of the uninvolved extremity. Temperature and perfusion also are compared with those of the uninjured limb. An expanding hematoma overlying the site of injury usually indicates significant arterial injury. In contrast, complete arterial transection or an intimal tear in a tight space may lead to minimal blood loss. The clinician must always determine the presence and quality of the peripheral pulses in both the injured and uninjured limbs but must exercise care in interpreting the findings. Pulses may remain palpable in the presence of arterial injury due to collateral flow or pulsatile fluid wave transmission. Conversely, absent pulses may reflect transient arterial spasm that alone has little significance (2). However, the finding of diminished or absent pulses in the presence of an extremity injury should never be attributed to arterial spasm without a complete evaluation.
When necessary, emergency angiography should be performed. For upper extremity injuries, an Allen test can be done to assess arterial inflow to the hand. The child must cooperate by raising and clenching his or her hand. The clinician tightly compresses both the radial and ulnar arteries until the palmar surface is blanched. The hand is lowered and opened, and then one vessel is released and reperfusion of the hand observed. The test is repeated, releasing the other artery and comparing the difference in reperfusion time. Normally, both the radial and ulnar arteries adequately reperfuse the palmar surface of the hand in less than 3 seconds. The Allen test should also be performed in the uninvolved limb for comparison.
Sensation should be assessed by localizing light touch using a cotton wisp. Two-point discrimination is done using the blunt ends of a paper clip, one centimeter apart. It may not be possible to reliably assess sensation by either of these
methods in a nonverbal or uncooperative child. Still, the clinician should almost never use painful discrimination for the examination of the young child, as it is itself painful and rarely provides useful information (10,11). It is still possible to assess sensation in the hand of the infant or fearful child. Because autonomic nerve fibers travel in nerve bundles, the wrinkle test can be done to assess sensation to the hand. The hand is first submerged in warm water for 5 to 10 minutes. The hand and fingers are then inspected for wrinkling. Those areas with impaired sensation will not wrinkle (10).
methods in a nonverbal or uncooperative child. Still, the clinician should almost never use painful discrimination for the examination of the young child, as it is itself painful and rarely provides useful information (10,11). It is still possible to assess sensation in the hand of the infant or fearful child. Because autonomic nerve fibers travel in nerve bundles, the wrinkle test can be done to assess sensation to the hand. The hand is first submerged in warm water for 5 to 10 minutes. The hand and fingers are then inspected for wrinkling. Those areas with impaired sensation will not wrinkle (10).
Motor function is next evaluated in both the injured and uninjured extremities. Function and strength are carefully assessed and compared. In the evaluation for compartment syndrome, it is imperative to passively stretch and have the child actively flex the limb and the muscle group of concern. For example, the anterior compartment of the leg is the most common site for development of compartment syndrome. The primary nerve supply is via the deep peroneal nerve that serves in foot dorsiflexion by innervating the tibialis anterior and extensor digitorum longus muscles. In the event of compartment syndrome, these muscles are weakened and active foot dorsiflexion and toe extension are impaired. Furthermore, passive flexion of the toes results in severe pain due to ischemia resulting from increased intracompartmental pressure. Once the examination is complete, anesthesia (local, regional, or general) should be provided as necessary before radiographs are obtained or the wound is explored.
In the special case of the obtunded and/or comatose child, the physician must have a higher index of suspicion for neurovascular impairment and consequently a lower threshold for intervention (e.g., measuring intracompartmental pressures) (12). Any orthopaedic injury that leads to neurovascular compromise represents a potentially limb-threatening emergency. Insufficient arterial blood flow to a distal extremity can result from either vessel transection or laceration caused by a bony fragment or projectile missile (e.g., gunshot wound). Arterial spasm or compression caused by extrinsic pressure from the fractured bone or edematous tissue also may lead to ischemic damage. Venous obstruction is usually the result of tissue swelling from the injury or from tightly applied circumferential casts or dressings. If unrecognized or untreated, this can also lead to the development of compartment syndrome (12).
Nerve injury may be temporary due to stretching, and nerve dysfunction generally resolves within 6 months. However, permanent dysfunction can result from ischemic necrosis in late compartment syndrome. Nerves, like vessels, also can be transected. It is therefore essential to perform a rapid, accurate assessment and appropriate intervention to prevent permanent functional impairment or loss of the involved limb.
The proximity of the brachial artery to the elbow, the proximity of the popliteal artery to the knee, and the proximity of the pudendal artery to the pelvis make these three areas (elbow, knee, pelvis) particularly vulnerable to vascular compromise following a significant injury. The radial, median, and ulnar nerves around the elbow and the tibial and common peroneal nerves at the knee are at relatively high risk for nerve impairment. Therefore, much attention will be focused on recognizing and treating supracondylar humeral fractures and severe knee injuries. It must be stressed, however, that any complicated fracture or dislocation may be unstable and lead to associated vascular ischemia or nerve damage. A brief overview of unstable pelvic, femoral shaft, and complex tibia-fibula and ankle fractures is included. Features that aid in the prompt recognition, diagnosis, and management of compartment syndrome are highlighted first.
Compartment Syndrome
Pathophysiology
Generally speaking, insufficient blood flow to an extremity may lead to ischemic damage as early as 4 hours after an injury (3,13,14). Irreversible damage can occur 6 to 12 hours after onset of compromised vascular perfusion (4,11,15,16).
Compartment syndrome can be caused by any condition that leads to elevated tissue pressure within muscle groups enveloped by fascial sheaths. The actual incidence of compartment syndrome in children is unknown, but it is safe to say that it is a rare entity (12). Still, due to the potentially grave consequences if unrecognized and untreated, it is prudent to consider the diagnosis of compartment syndrome when faced with an extremity at risk.
The pathophysiology of compartment syndrome has been well studied (3,4,12,13,14,15,16,17,18,19,20,21,22). The increased pressure within the confined space leads to restriction of blood flow distally, with subsequent ischemic insult to the extremity. It was previously believed that the ischemia leading to compartment syndrome was secondary solely to impaired arterial blood flow. Acute compartment syndrome, however, is known to occur in the presence of palpable distal arterial pulses. Current theory holds that it is narrowing of the arterial-venous pressure gradient, either by increased venous pressure or decreased arterial pressure, that causes hypoperfusion and ischemic injury. Subsequent reperfusion within the compartment leads directly to elevated pressure from increased flow in the restricted space and indirectly to increased pressure from local tissue edema. Further ischemia develops, leading to necrosis and limb dysfunction.
A myriad of conditions may lead to compartment syndrome (3,12). Again, the basic principle is either (a) the contents within the confined sheath unduly swell or (b) the envelope surrounding the muscle group is overly constricting. Bleeding directly into the compartmental space secondary to blunt trauma (fractures, contusions), penetrating trauma (gunshot wounds) with arterial injury, primary limb surgery, joint dislocations, or fracture reduction may lead to the development of compartment syndrome. Excessively tight MAST suits, casts, air splints, and dressings may lead to compartment syndrome by excessive external constricting pressure. Intraosseous infusions, snakebites, burns, and cardiac catheterizations may result in an increased compartmental volume
from intravenous fluid infiltration or capillary leak and are known causes of compartment syndrome in children.
from intravenous fluid infiltration or capillary leak and are known causes of compartment syndrome in children.
A chronic or recurrent form of compartment syndrome may develop from prolonged, excessive muscle use. Its pathogenesis is not well understood, and the physician must have a high clinical suspicion for the diagnosis. If this entity is being considered, acute management involves cessation of exercise and referral to an orthopaedic specialist (3,4). Supracondylar fractures of the humerus, combined radius and ulnar fractures, femur fractures with skin traction, and tibial fractures are the most common mechanisms of injury leading to compartment syndrome in children (12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree