Appendix and Meckel’s Diverticulum
Robert S. Sawin
University of Washington, Children’s Hospital and Regional Medical Center, Seattle, Washington 98105-0371.
APPENDIX
Anatomy
Embryology
The appendix develops from the cecum, which first appears during the fifth week as a ventral enlargement of the midgut. The appendix is first visible at 8 weeks, apparently the result of disproportionately slow growth of the terminal cecum compared with the rest of the hindgut. Although the appendix does continue to grow, its diameter is only 20% to 25% of the diameter of the cecum at birth. Absence of this expected asymmetric growth may explain the few documented patients with congenital absence of the appendix. The asymmetric growth of the appendix and cecum also causes the appendix to shift from the apex of the cecum to a more medial position near the ileocecal valve. The variability of this shift results in multiple possible positions of the appendix.
Gross Anatomy
In addition to the variable location of the tip of the appendix, its relationship to surrounding structures is protean. The appendix may lie across the psoas muscle or over the pelvic brim, resting on the pelvic fascia that overlies the obturator internus muscle. These positions account for the physical findings of pain on extension of the hip (psoas sign) or pain with flexion and internal rotation of the thigh (obturator sign). Additional variations in appendiceal position result from the abnormalities of midgut rotation. Because the attachment of the appendix to the base of the cecum is a constant, anomalies such as malrotation can lead to a left-sided appendix. The size and shape of the appendix also vary. It may be funnel shaped or cylindrical with a uniform caliber. The length can range from 0.3 to 33 cm, with appendices of males tending to be slightly longer than those of females. The diameter is typically less than or equal to 6 mm, and thus a measurement larger than that is one of the ultrasound criteria for diagnosis of appendicitis.
The arterial supply to the appendix is from the ileocolic artery, a branch of the superior mesenteric artery. One of four terminal branches of the ileocolic artery, the appendiceal artery passes posterior to the terminal ileum and gives off multiple short, straight branches to the appendix. The retroileal course of the appendiceal artery may predispose it to kinking with resultant ischemia and inflammation of the appendix. The venous drainage of the appendix is via the superior mesenteric vein to the portal vein, which accounts for the occasional findings of pylephlebitis or liver abscess following appendicitis (1).
Like the rest of the midgut, the appendix is innervated by branches of the splanchnic nerves that arise from the lower thoracic ganglia. Typically, the T10 ganglion is the one via which painful stimuli from the appendix are conducted to the dorsal nerve root, along the spinothalamic tract to the brain. Because the umbilical region of the abdominal wall develops from the same embryonic region, or dermatome, as the appendix, this explains in part why appendiceal pain initially localizes to the periumbilical region. This innervation is common to the kidney, upper ureter, and testicle, accounting for some of the organs that must be considered in the differential diagnosis of periumbilical pain.
Histology
Like the rest of the intestinal tract, the appendix has four layers. In both the mucosa and submucosa, germinal follicles and lymphoid pulp are prominent in infants and children. The lymphoid tissue gradually atrophies with age. Whether this lymphoid tissue predisposes children to appendicitis by luminal obstruction during periods of inflammation is unproved.
Diseases
Appendicitis
Epidemiology
The risk of developing appendicitis has been estimated as 6% to 10% during an average lifetime. Residents of Third World countries have a substantially reduced risk of developing appendicitis compared with those in developed nations. A diet high in sugar, low in fiber content, and good hygiene with a resultant decreased exposure to enteric pathogens at an early age have all been hypothesized as risk factors for appendicitis. The epidemiologic literature, however, contains contradictory data. The risk of developing appendicitis is lowest in infancy, perhaps because of the relatively wide base of the appendix at that stage of development. Approximately 1% of all children younger than 15 years of age develop appendicitis with a peak incidence between 10 and 12 years of age (2).
The risk of developing appendicitis that progresses to perforation is greater in children than in adults. In published series of appendicitis from children’s hospitals, the incidence of perforation is 20% to 76% but typically is approximately (3). This may be a consequence of the difficulty in making the diagnosis of acute appendicitis in the toddler or preschool-age child who cannot communicate as effectively as the older child. An additional factor is the unfortunate tendency of parents and physicians to attribute all childhood fevers and gastrointestinal symptoms to viral illnesses. There is also some evidence to suggest that easy access to health care providers may reduce the risk of perforation because managed care patients with private insurance have lower perforation rates than Medicaid or uninsured patients (4).
Pathophysiology
The pathologic sequence ending in appendicitis is believed to be analogous to that seen with cholecystitis. Although the causes of appendicitis and cholecystitis may be multiple, they often have in common obstruction of the proximal lumen. The role of obstruction in appendicitis was established by Wangensteen in an elegant experiment where the symptoms of appendicitis were replicated by ligating the base of an exteriorized appendix. Fecaliths are the most frequent example of obstructing appendiceal lesions and are present in approximately 30% to 50% of appendicitis patients (5). Other obstructing lesions may include lymphoid hyperplasia, foreign bodies, parasitic infections, or conditions that cause increased colonic pressure and decreased motility such as Hirschsprung’s disease or meconium ileus. After the obstruction, the mucosa continues to secrete mucous, resulting in intraluminal pressure increases that lead to venous congestion and edema. The intramural pressure increases until ischemia and tissue acidosis of the appendiceal wall result. Finally, mucosal ulceration is followed by bacterial invasion leading to invasive infection of the appendix.
Although this orderly pathologic sequence is consistent with the progressive history and symptoms of appendicitis, the role of obstruction in the pathogenesis of appendicitis is somewhat equivocal. Likewise, the absence of fecaliths in 50% to 70% of the appendicitis patients supports the etiologic role of factors other than obstruction.
Microbiology
The possible role of viral infections in appendicitis has been implicated by the frequent prodrome of symptoms with which children with appendicitis may initially present. In addition, appendicitis has been described following Varicella infections. Cultures of mesenteric lymph nodes and resected appendices may grow adenovirus in children with appendicitis. It is hypothesized that the viral illness may result in lymphoid hyperplasia or lymphadenopathy, which can obstruct the lumen. In addition, the viral illness may result in dehydration leading to a higher likelihood of inspissated stool or mucous leading to fecalith formation and obstruction.
Enteric bacteria are the most common organisms associated with appendicitis. In the patients with perforation, Escherichia coli, Enterococcus, Bacteroides, and Pseudomonas are the species most frequently isolated from the abscess. Whether these are the main pathogens in nonperforated appendicitis is not known. Parasitic infections with Enterobius or Ascaris have also been reported in association with appendicitis. These organisms may cause a local inflammation or may contribute to luminal obstruction leading to bacterial invasion and supurative appendicitis.
Pathology
In the early stages of acute appendicitis, the appendix appears thickened and feels turgid, with increased serosal vascularity. The distal portion of the organ is often distended, especially when an obstructing lesion such as a fecalith is present. Histologically, the mucosa is ulcerated and infiltrated with inflammatory cells. As the bacterial invasion progresses, the inflammatory infiltrate progresses from the mucosa through the muscularis. Cloudy peritoneal fluid filled with polymorphonuclear cells but lacking bacteria may be seen as the inflammation progresses. Necrosis of all layers, or gangrenous appendicitis, may occur with or without perforation. If perforation has occurred, cloudy, foul-smelling peritoneal fluid is found. Usually, a polymicrobial flora can be cultured from this fluid. Microscopically dense sheets of polymorphonuclear leukocytes and erythrocytes are seen in the lumen, muscularis, and mesoappendix. The site of perforation may be difficult for the pathologist to identify, especially if the appendix is surrounded by the omentum.
TABLE 80-1 Symptoms of Appendicitis. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Clinical Presentations
Acute Appendicitis
The child with appendicitis may present with many different symptoms, making appendicitis the most commonly misdiagnosed surgical lesion in the United States. The most frequent symptoms are listed in Table 80-1. The constellation of abdominal pain, fever, anorexia, and nausea is classically present in children with appendicitis. Unfortunately, fewer than one-half of the children with acute appendicitis present with this complete spectrum of symptoms. The orderly sequence of pathophysiologic events described previously usually causes a characteristic progression of symptoms. This sequence typically begins with vague abdominal pain apparently originating in the periumbilical region. This vague localization of pain is termed referred pain. The entire midgut shares the same T10 dermatome with the umbilicus so afferent pain stimuli are erroneously interpreted by the brain as originating in the umbilicus. Thus, any painful lesion or distension in the intestinal tract will cause a similar periumbilical pain. As the serosa of the appendix becomes inflamed, it begins to cause local inflammation of the adjacent peritoneum. With this peritoneal irritation, the pain is usually more localized to the right lower quadrant, thus manifesting itself as migration of pain to the right lower quadrant. As the inflammation and distention of the appendix progresses, the pain worsens. If the appendix is not removed at this time, then the swelling progresses and culminates in perforation. Thus, physical examination becomes especially important.
Examining the child with abdominal pain requires a calm, nonthreatening demeanor and careful observation. The child’s appearance can be very revealing because the appendicitis patient will lie quietly, often with the knees drawn up, and resist movement in any way. Shaking of the bed or stretcher and having the patient cough may elicit wincing or complaint. Asking the child to get off the bed and walk can be informative. The child with early appendicitis may move reasonably comfortably, but will complain of pain when asked to walk on his or her heels or to jump to touch the examiner’s hand held high. Having the child move from a supine to a sitting position or vice versa may also elicit discomfort.
Examination should begin with all other parts of the body. Auscultation of the lungs is important for pneumonia and can present as abdominal pain. Pain with external rotation of the thigh (obturator sign) or extension of the hip with the child in a left lateral decubitus position (psoas sign) indicates peritoneal irritation, often due to a retrocecal appendicitis. Before examining the abdomen, the examiner’s hands should be warmed. Many surgeons ask the child to point with one finger to the spot that hurts the most. With the knees bent to relax the abdominal muscles, the examination should begin far away from the area of maximal tenderness. Younger children may be more willing to allow palpation if the examiner places the patient’s hand or the stethoscope on the abdomen and palpates “through” these less threatening objects. Distraction by asking the child questions about their family, school, favorite activities, and so on, during the examination is very helpful. Close inspection of the facial expression is more discriminating than asking whether each maneuver hurts. If good relaxation can be accomplished, rectus muscle spasm can be appreciated. Sudden withdrawal of the palpating hand to check for rebound tenderness is startling for children, and therefore, not as reliable a physical sign as in adults. Palpation of the left lower quadrant eliciting right lower quadrant pain, termed Rovsing’s sign, is a fairly specific sign for appendicitis. If diffuse tenderness and guarding are present, free perforation is likely, especially in children younger than 6 years of age. The rectal examination is considered most unpleasant by the patients and by some physicians, and is consequently often avoided. Although it should be deferred until last, it is occasionally one of the most important parts of the examination, particularly in the patient who does not have clear anterior abdominal wall tenderness, such as those with retrocecal appendicitis. The child should be gently advised that this exam will feel strange, but may not hurt. The left lateral decubitus position with the knees drawn to the chest works well. Once the discomfort of the examining finger is tolerated by the patient, the exam should begin away from the right pelvis, leaving it for last. Induration or focal tenderness in the right or midline pelvis are suggestive of appendicitis. If perforation has already occurred, a mass may be palpable on rectal exam, suggesting the option of operative transrectal drainage. It is also helpful to palpate posteriorly. This may differentiate pain due to peritoneal irritation from discomfort due to the examination alone.
Perforated Appendicitis
As mentioned previously, appendicitis leading to perforation is more common in children than in adults. The large diameter relative to the cecum and thin wall of the appendix in childhood may be contributing factors that predispose children to a more rapid progression of the disease. A more likely explanation of the higher perforation rates is the delay in presentation and the delay in diagnosis. Parents are prone to assuming that any gastrointestinal
symptoms are related to “the flu” or to “something he or she ate,” and thus are often slow to call the physician. Physicians frequently compound the problem with similar rationalization. Indeed, several studies have indicated that the major source of delays in making the diagnosis of appendicitis can be attributed to the health care provider (6).
symptoms are related to “the flu” or to “something he or she ate,” and thus are often slow to call the physician. Physicians frequently compound the problem with similar rationalization. Indeed, several studies have indicated that the major source of delays in making the diagnosis of appendicitis can be attributed to the health care provider (6).
The paradigm of the pathophysiologic sequence of appendicitis discussed previously is a convenient way to assist in making the diagnosis. It is reliable only if an accurate history is obtained and if the patient is examined sequentially. Because the goal is to make the diagnosis of acute appendicitis before perforation, the key period is the first 24 hours, for the risk of perforation within 24 hours of the onset of symptoms is less than 30%. Conversely, if symptoms have been present for more than 48 hours, the probability of perforation is greater than 70%. This paradigm is less useful in the children younger than 5 years of age because their disease history, physical signs, and symptoms are more difficult to assess and the diagnosis more difficult to make.
The symptoms of perforated appendicitis evolve from a transient decrease in the abdominal pain secondary to the release of pressure in the appendix, to a severe generalized abdominal pain, fever often higher than 38°C, worsening anorexia with nausea and vomiting, dehydration, and diarrhea (which can be misleading by suggesting the diagnosis of gastroenteritis). Occasionally, the child will progress through these symptoms and manage to localize the perforation by “walling off” the infection between the surrounding viscera and the omentum. This is particularly true of the retrocecal and retroileal appendices. Younger children with their veillike omentum are less capable of this localization and are more likely to have generalized peritonitis. The other physical findings in most children with perforated appendicitis are similar to those found in nonperforated appendicitis. The absence of bowel sounds or the presence of high-pitched, tinkling bowel sounds may suggest the presence of a small bowel obstruction, particularly in the children with retroileal appendicitis and those younger than age 6.
Appendiceal Mass
Appendicitis with a palpable mass is usually a consequence of perforation. The mass may be an abscess or a phlegmon, lacking frank pus and composed of omentum and matted loops of bowel. The management of patients who present with abdominal masses in the setting of appendicitis is controversial. Emergency surgery for such patients is seldom indicated because most will benefit from fluid resuscitation and initiation of broad-spectrum intravenous antibiotics before intervention. Laparotomy with drainage of the abscess, if present, and appendectomy has been the standard approach. Opponents of such an approach argue that the acute inflammation of the cecum and surrounding bowel can result in long, difficult operations with more blood loss, increased risk of bowel injury, and inability to safely perform a complete appendectomy.
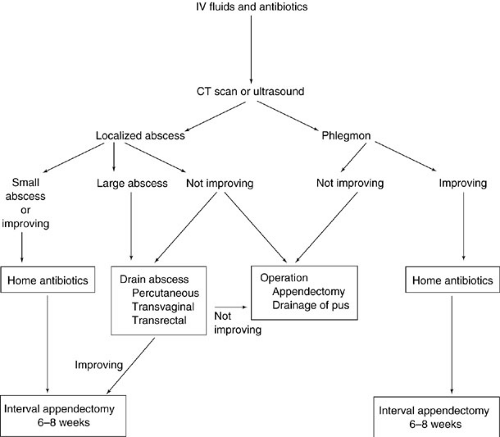
Alternative approaches depend on whether the mass is an abscess or phlegmon, a distinction that may require ultrasonic or computed tomography (CT) scan imaging to resolve. Figure 80-1 shows an algorithm for the delayed laparotomy approach recommended by some pediatric surgeons. If the mass is determined to be a phlegmon, then intravenous antibiotics and volume resuscitation without immediate laparotomy is a safe and effective treatment, provided the patient shows clinical improvement with lower temperature spikes, decreasing leukocytosis, and decreasing abdominal tenderness. The elective interval appendectomy can be performed 6 to 8 weeks later. If clinical improvement does not occur after 12 to 24 hours of this nonoperative management, then laparotomy is indicated. If an abscess is identified, drainage by percutaneous, transvaginal, or transrectal routes is an effective treatment after resuscitation and intravenous antibiotics. Small abscesses (less than 2 cm in diameter) may be managed with antibiotics alone. As long as clinical improvement occurs, then antibiotic therapy is continued until the patient is afebrile, the white blood cell count is normal, and the abdominal tenderness is resolved. This postdrainage therapy can be completed as an outpatient, thus reducing the length of hospital stay. Interval appendectomy 6 to 8 weeks later is usually remarkably uncomplicated with a surprising absence of inflammatory adhesions and a postoperative hospital stay of 1 day. Whether this delayed operative therapy is superior to immediate appendectomy remains to be proven. One prosepective, nonrandomized study suggests that delayed surgical treatment is associated with a longer total length of stay and a higher morbidity rate (7). The question requires a controlled, randomized, prospective study.
Recurrent Appendicitis
There is some debate about the existence of recurrent or chronic appendicitis. If the paradigm of progressive pathophysiologic changes discussed earlier in this chapter is accepted, then it is difficult to reconcile the possibility of appendicitis resolving without some therapeutic intervention. Nonetheless, approximately one-fourth of patients with surgically proven acute appendicitis report a history of prior episodes of abdominal pain that are similar to the ones that prompted the appendectomy. Histopathology of resected appendices may show both chronic and acute inflammatory infiltrates and fibrosis, implicating prior episodes of acute appendicitis. In addition, as many as 60% of patients who are successfully treated in a nonoperative fashion for perforated appendicitis will have abdominal symptoms suggestive of recurrent appendicitis prior to the interval appendectomy. The histopathology of the interval appendectomy specimens may show acute, as well as the expected chronic, inflammation in such patients.
A more uncommon entity is chronic appendicitis. Children with chronic abdominal pain are difficult to evaluate because many have no apparent pathologic cause for their pain. There are a small number of such children who undergo extensive diagnostic testing, including abdominal ultrasound and gastrointestinal contrast studies. Some of these patients have thickening of the appendix seen on ultrasound or no filling of the appendix seen on barium enema. These findings in the setting of chronic abdominal pain warrant an appendectomy and will frequently result in resolution of the symptoms. The diagnosis of chronic appendicitis is inferred, regardless of the absence of histopathologic confirmation. This absence of histologic verification prompts many physicians to attribute the symptomatic improvement to a placebo effect. Like postcholecystectomy syndrome and irritable bowel syndrome, chronic appendicitis may be difficult to define and diagnose, but there are patients whose disease appears to fit the diagnosis (8).
Special Considerations
Infants
Appendicitis in neonates is very uncommon and has a mortality rate of 50% to 80%. The diagnosis is often not recognized until postmortem. If identified in the neonatal period, appendicitis should be treated with emergent resection and rectal biopsies for frozen section diagnosis because Hirschsprung’s disease is frequently the underlying cause. Colostomy at the level of ganglionated bowel is the only safe option in those patients with documented Hirschsprung’s disease and appendicitis. Postoperative care in the neonatal intensive care unit with broad-spectrum antibiotics is typically warranted.
Appendicitis in children between 1 month and 1 year of age is unusual. These children are likely to present with perforated appendicitis and to manifest the complications of appendicitis, such as intestinal obstruction and systemic sepsis. It is indeed rare and fortuitous to make the diagnosis of acute appendicitis before perforation in this age group. This is a serious illness in infants and has a mortality rate as high as 10%.
Postpubertal Females
An accurate diagnosis of the source of abdominal pain in postpubertal girls is often difficult. In addition to appendicitis, ovarian pathology such as an ovarian cyst ruptured (or intact), ovarian torsion, pelvic inflammatory disease, and pain with ovulation, or mittelschmerz, can be common causes of severe lower abdominal pain. In most series, the rate of appendicitis resulting in perforation is no higher in this group of patients, but the rate of “negative
appendectomies” (i.e., appendices lacking histologic evidence of inflammation) is reported to be as high as 40%. The liberal use of ultrasound examinations may result in lower negative appendectomy rates because ultrasound can be valuable to assess both the ovaries and the appendix. Diagnostic laparoscopy has been touted as an effective way to define the cause of lower abdominal pain in this age group. There is some debate, however, as to whether this avoids “unnecessary appendectomies” because many surgeons reason that the appendix should be removed during the laparoscopic procedure, regardless of its gross appearance.
appendectomies” (i.e., appendices lacking histologic evidence of inflammation) is reported to be as high as 40%. The liberal use of ultrasound examinations may result in lower negative appendectomy rates because ultrasound can be valuable to assess both the ovaries and the appendix. Diagnostic laparoscopy has been touted as an effective way to define the cause of lower abdominal pain in this age group. There is some debate, however, as to whether this avoids “unnecessary appendectomies” because many surgeons reason that the appendix should be removed during the laparoscopic procedure, regardless of its gross appearance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree