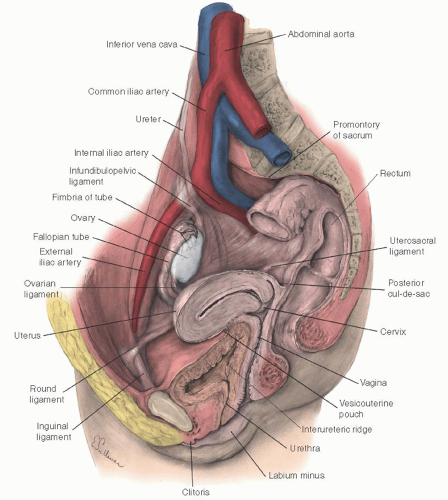
Genitourinary fistulas can be congenital or acquired. Congenital fistulas are exceedingly rare, with only a few case reports in the literature. Most reported cases are associated with other urogenital anomalies. Acquired fistulas may be the result of childbirth, pelvic surgery, malignancy, irradiation, infection, and an assortment of unusual presentations (
Table 43.2). This section discusses obstetrical and nonobstetrical urogenital fistulas. The nonobstetrical fistula portion will further discuss the following issues associated with urogenital fistula etiology and epidemiology: hysterectomyassociated cystotomies, urogynecologic and other gynecologic procedures, urethrovaginal fistulas, and an assortment of miscellaneous circumstances that have been reported in the literature.
Obstetrical Urogenital Fistulas
In the developing world, the most common predisposing factor is prolonged, obstructed childbirth, accounting for over 80% of genitourinary fistulas in these regions. These fistulas develop from the resulting necrosis of the vaginal and bladder wall and, therefore, are large and often associated with other related damages. These circumstances have become exceedingly rare in developed countries owing to improvements in access to and delivery of skilled obstetric care. Ibrahim et al. report that the mean age at diagnosis was 15 years, patients were in labor for 4 days on average, and the fetus died in 87% of cases. These findings are similar to another single-institution series from Nigeria, where 79% of fistula patients had labors that lasted over 2 days, and 21% had been in labor for 4 or more days during the index pregnancy associated with the injury. The fetal fatality rate in these cases was 92%. In a rural, population-based study of parturients in Senegal, the incidence rate was 124 per 100,000 deliveries, whereas no fistulas were noted in six major cities in West Africa. It is estimated that over 33,400 new cases of obstetric fistulas occur annually in sub-Saharan Africa, with an incidence of over 120 per 100,000 births. Constitutional risk factors include short stature, lower
education and socioeconomic levels, and young maternal age. Circumstantial contributing factors include a very high rate of home births attended to by unskilled birth attendants, wherein only 35% of deliveries in Nigeria are attended by trained personnel. Late presentation in northern Nigeria is primarily related to lack of husband’s permission followed by transportation difficulties (distance, nonavailability of vehicle, and bad roads). The general lack of hospital resources including surgical supplies and reliable electrical power have been cited as contributing to delayed cesarean section for those who need it.
Hilton reviewed VVFs in developing countries and outlined management strategies to include immediate catheter drainage as long as the tract has not yet epithelialized, perhaps even prophylactically following obstructed labor with evident vaginal sloughing. Attention to adequate vulvar skin care, nutrition, lower extremity rehabilitation, and counseling are important adjuncts in the care of obstetrical fistula patients. Arrowsmith et al. similarly remind us of the spectrum of additional trauma sustained by the fistula patient and the need to address more than just the “hole in the bladder.” Additional concurrent injuries may include and result in amenorrhea, total urethral loss, stress incontinence, hydronephrosis, renal failure, anorectal injury, cervical destruction, vaginal stenosis, nerve damage affecting mobility, and other physical maladies. The psychosocial toll is equally devastating with divorce, separation from family and social support structures, worsening economic hardship, isolation, and malnutrition being commonplace.
Surgical correction is curative in the first operation for obstetric VVF in about 80% of cases. Multiple attempts may be necessary to achieve a success rate over 95% for large fistulas. As the reality and complexity of this global tragedy is realized, solutions are suggested for its prevention that include improving the stature of women and access to competent routine and emergency obstetrical care as well as family planning services. Both global and regional economic and political actions will be necessary to effect meaningful and lasting change.
Other obstetric events and procedures have been noted to be associated with urogenital fistula formation worldwide. Cesarean section, especially in patients who have had prolonged, obstructed labor, may result in formation of a vesicouterine fistula (Youssef syndrome). The classic presentation is that of cyclical hematuria (menouria), amenorrhea, and urinary continence, but it may also present with total urinary incontinence with normal menstruation and represents 1% to 4% of all genitourinary fistulas. Vesicovaginal fistulas have also been reported following cesarean section, with or without associated hysterectomy. Even more rarely reported are vesicocervical, urethrovaginal, ureterovaginal, and ureterouterine fistulas following cesarean section. Operative vaginal delivery may rarely result in vesicouterine fistula in a patient undergoing vaginal birth delivery after cesarean section. Similarly, cervical cerclage placed for the treatment of cervical insufficiency has been associated with vesicovaginal and vesicocervical fistulas. A recent case series and pooled analysis suggests that previous cervical procedures (prior cerclage or cervical conization), prior cesarean delivery, and use of the McDonald technique (which does not involve a bladder dissection as with the Shirodkar technique) may play a role in the formation of urogenital fistula in these cases.
Although not an obstetrical practice, the traditional tribal practice of “gishiri cutting” is associated with formation of urogenital fistulas and is common in many parts of northern Nigeria. It involves cutting the anterior vagina with a razor or knife blade and has been noted to be the primary cause of fistula formation in 13% to 15% of cases. In more recently reported series in areas where gishiri cutting was reported, urogenital fistula was noted in 2.3% to 6.2% of cases.
Nonobstetrical Urogenital Fistulas
Nonobstetric urogenital fistulas have been reported as a consequence of gynecologic, urologic, and general surgical procedures and are the most commonly seen in developed countries. Various manifestations of urogenital trauma, certain medical conditions, and even medication instilled into the bladder have also been associated with subsequent development of urogenital fistulas.
Hysterectomy is the most common surgical procedure associated with a VVF. In a retrospective review of the Mayo Clinic experience regarding the treatment of 303 women with genitourinary fistulas, 190 were vesicovaginal. Of these, 156 (82%) were associated with hysterectomy (mostly abdominal), 19 (10%) with cesarean section or forceps delivery, and 6 (3%) each with radiation and with trauma.
Harkki-Siren et al. reviewed the incidence of urinary tract injuries from a Finnish national database of 62,379 hysterectomies with a VVF prevalence of 0.8 per 1,000. In a nationwide registry-based cohort study from Sweden that included all hysterectomies performed for benign indications over 30 years (
n = 182,641), the prevalence of surgically managed genitourinary fistulas was 1.1 per 1,000. This is the same rate reported in a retrospective study of two institutions in the
United States but has been reported as high as 3.0 per 1,000 in one of those institutions.
In a retrospective cohort study of 343,771 hysterectomies in the English National Health Service from 2000 until 2008, the overall urogenital fistula (vesicovaginal and urethrovaginal) rate was reported to be 1.3 per 1,000 within the year following surgery. Rates were noted to vary by the procedure type and the indication, with the highest rate in patients undergoing radical hysterectomy due to cervical cancer (11.5 per 1,000) and the lowest for vaginal hysterectomy due to prolapse (0.26 per 1,000). Most interesting was a subanalysis of abdominal hysterectomy for benign conditions excluding prolapse, where a 46% increase in the risk of fistula rates was noted over the course of the study itself from 1.5 per 1,000 in the first 2 years to 2.2 per 1,000 in the last 2 years of the study. See
Table 43.3 for a summary of the various epidemiologic studies discussed.
As previously noted, hysterectomy for cancer is associated with an increased risk of fistula formation. In a retrospective study of 536 patients who underwent a radical hysterectomy due to cervical cancer, a VVF rate of 2.6% and a ureterovaginal fistula rate of 2.4% were reported. Risk factors included cancer stage, intraoperative bladder injury, diabetes, and postoperative surgical site infection. Vaginal cancer shows a high likelihood of fistula development (both vesicovaginal and rectovaginal) with no association to radiation therapy.
Hysterectomy-Associated Cystotomy and Vesicovaginal Fistula Formation
The association of a cystotomy sustained at the time of hysterectomy (recognized or not) and subsequent development of a VVF has long been noted but is nonetheless complex (see further discussion under Pathogenesis). A review of published articles reporting on cystotomies during gynecologic surgery showed that the rate of cystotomy is significantly increased in series that employed routine cystoscopy rather than the traditional intra- or postoperative diagnosis on which the other studies relied. These disparate rates of bladder injury continue with two distinct single-institution studies that employed routine cystoscopy at the time of hysterectomy, reporting a cystotomy rate of 2.6% to 2.9%, while estimates from a US national hospital discharge database-derived study reported bladder injury rates of only 0.7% to 1.4% depending on the type of hysterectomy. Although one cannot conclude that the two- to threefold difference in cystotomy rates is due to detection bias, it is not unreasonable to consider that some bladder injuries may go undetected during hysterectomy, which may contribute to the overall posthysterectomy VVF rate.
Even when a cystotomy is immediately recognized and repaired, a subsequent VVF may still occur. In their data on over 43,000 total abdominal hysterectomies, Harkki-Siren et al. report failure of primary bladder repair 18% of the time. In a case-control single-institution study of 1,317 benign hysterectomies, which included routine cystoscopic evaluation, 34 cystotomies were recognized and repaired, but among those, four patients (11.8%) developed a VVF. The degree of bladder injury retrospectively assessed by using the American Association for the Surgery of Trauma (AAST) system was found to be strongly associated with subsequent fistula formation despite cystotomy repair. The AAST grading system is used to describe the degree of injury to the bladder using the location and size of the defect with higher grades denoting worse trauma. Of highest risk are larger extraperitoneal (>2 cm) injuries, any intraperitoneal bladder injuries, and injuries involving the bladder neck or the trigone. Additional associated risk factors for occurrence of a VVF following cystotomy and repair include larger uterus, longer operative time, and longer hospital stay. A trend was noted when the hysterectomy was associated with greater than 1 L blood loss and for tobacco use. The strong association of bladder injury severity with subsequent VVF formation persisted in a subsequent expansion of this original study. There were 5,698 hysterectomies in the combined cohort where 102 cystotomies were diagnosed and repaired. Of these, there were six VVFs (5.9%) identified.
Urogynecologic Procedures
Due to the immediate proximity of the lower urinary tract to the operative field in surgeries for the treatment of urinary incontinence, pelvic organ prolapse, and other pelvic floor disorders, there have been reports of both vesicovaginal and urethrovaginal fistulas with these types of surgery. Surgeries for the treatment of stress urinary incontinence have been implicated, including Burch colposuspension, bone-anchored cystourethropexy, Stamey bladder neck suspension, transobturator tape, and retropubic tension-free vaginal tape procedures.
Surgery for anterior prolapse occurs adjacent to the bladder and may result in immediate or delayed injury to the lower urinary tract. Anterior colporrhaphy has been associated with VVF only in case reports regarding fistula repairs. In a single surgeon case series of 519 anterior colporrhaphy patients (with Kelly-Kennedy urethropexy for the treatment of stress incontinence), the authors reported two urethrovaginal fistulas but no VVFs. Mesh kit procedures for the treatment of anterior prolapse have been associated with VVFs by several investigators. The incidence of VVF from trocar-based mesh kit surgery used for the treatment of anterior prolapse has been noted to be 0.3% in a large series. A recent case report noted a ureterovaginal fistula 28 days following a transobturator mesh kit surgery for anterior prolapse. A case report of a VVF following total abdominal hysterectomy with sacrocolpopexy for uterine prolapse noted that the fistula was adjacent to the distal edge of the colpopexy mesh.
Urethrovaginal fistulas have been associated with each of the various forms of midurethral (mesh) slings, vaginal urethropexies, and even periurethral bulking injections. With the increasing popularity of midurethral slings for the treatment of stress incontinence, reports of associated urethral injury and urethrovaginal fistulas have been accumulating. A literature review identified 3 VVFs and 11 urethrovaginal fistulas with retropubic and transobturator approaches implicated in both fistula types. The reviewed literature was insufficient to estimate the rates of urethrovaginal fistulas.
Urethrovaginal fistulas may occur as a consequence of urethral diverticulum repair. Review of available series, ranging from 18 to 85 patients each, reveals that urethrovaginal fistula develops in 1.2% to 7.8% of patients undergoing vaginal repair of a urethral diverticulum.
Other Gynecologic Procedures
Additional gynecologic procedures associated with urogenital fistulas, although with greater rarity (see
Table 43.2), include myomectomy, loop excision of the cervical transformation zone for cervical intraepithelial neoplasia, and voluntary interruption of pregnancy. Fistulas are noted following radiation therapy for gynecologic cancers and uterine artery embolization for leiomyomatous uteri.
Urethrovaginal Fistula
Urethrovaginal fistulas are less common than are VVFs, with an incidence ratio of 1 per 8.5. In the developed world, the most common predisposing event is surgery for urethral diverticulum, anterior vaginal prolapse and incontinence, radiation therapy, or trauma. Operative vaginal delivery and cesarean section also have been reported to precede urethrovaginal fistula formation. There has been an increase in urethrovaginal fistula formation related to the increased use of suburethral slings in the treatment of stress incontinence (please see subsection on Urogynecologic Procedures).
Miscellaneous Circumstances
An assortment of presentations and conditions can be found in the literature associated with urogenital fistulas mostly in the form of case presentations. These include Behçet syndrome; infections such as schistosomiasis, tuberculosis, lymphogranuloma venereum; endometriosis; accidental trauma; sexual trauma; masturbation; and a variety of retained foreign objects. Bladder calculi are found to be rarely associated with long-standing fistulas but not considered etiologic. Additional gynecologic associations include neglected diaphragm, neglected pessary, intrauterine device, and a ureteral wall stent used to treat a ureteral stricture. Intravesical instillation of mitomycin C has been reported to be associated with urogenital fistula formation.