Ventricular Shunt and Burr Hole Puncture
Ann-Christine Duhaime
James F. Wiley II
Introduction
Children with ventricular shunts can present to the emergency department (ED), office, or clinic with signs or symptoms of two major shunt complications—obstruction or infection. Shunt puncture, or “tapping” the shunt, refers to withdrawing cerebrospinal fluid (CSF) from the shunt apparatus percutaneously to aid in diagnosing a shunt malfunction or infection. In some cases, elevated intracranial pressure may be acutely relieved by removing some ventricular fluid. However, because shunt puncture carries an approximately 1% risk of introducing infection, and because improperly performed taps can damage certain types of shunt components, the physician should try to identify shunt problems without tapping the shunt when possible (1). Ideally, shunt punctures should be performed by a neurosurgeon who is familiar with the particular type of shunt in question and who has assessed the patient and can weigh the relative risks and benefits to be derived from a shunt tap. Because some shunt problems require immediate attention, however, the goal of this chapter is to review the basics of shunt anatomy and evaluation and to describe the indications and techniques for shunt puncture and burr hole puncture when a neurosurgeon is unavailable. Because of the potential for cardiorespiratory instability in the child with a shunt obstruction or infection, a physician should only perform this procedure in an area where monitoring, resuscitation equipment, and personnel are sufficient to handle sudden decompensation, such as in the ED, intensive care unit, or operating room.
Anatomy and Physiology
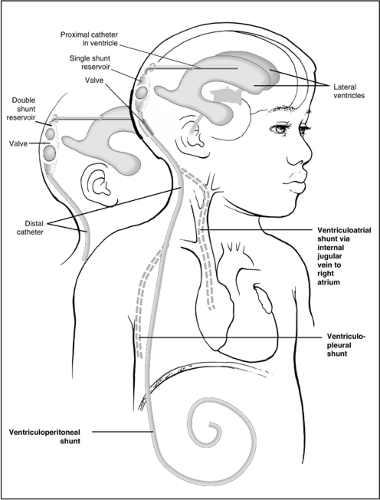
The majority of shunts placed for hydrocephalus consist of the following four main components (Fig. 42.1):
A proximal catheter, usually in the lateral ventricle
A distal catheter, usually in the peritoneal cavity or, less commonly, in the right atrium, pleural cavity, or other site
A reservoir, which is usually a single or double bubble or button, often located on the head near the burr hole entry site of the proximal catheter
A valve to control flow, which most often is a device located within or adjacent to the reservoir but in some cases is integrated into or part of the distal catheter (in-line or slit valve)
There are many types of valves, ranging from simple designs to more complex programmable valves that can be adjusted to increase or decrease resistance to flow by the application of an external magnetic device (Fig. 42.2).
Shunts are described according to their proximal and distal drainage sites; therefore, a ventriculoperitoneal (VP) shunt drains from the ventricle to the peritoneal cavity, a ventriculojugular (VJ) or ventriculoatrial (VA) shunt drains the ventricle through the jugular vein into the right atrium, and a lumboperitoneal (LP) shunt drains the lumbar subarachnoid space into the peritoneum. In addition, shunts have “brand names,” which often identify the manufacturer and the model of the shunt. As the number of valves available has increased, the terminology can be quite confusing. Clinicians can often sort out which valve is present in the patient by asking the family, by checking the operative report, or by looking at the plain films (shunt survey). Most major manufacturers have websites on which specific valve types are pictured; by searching these sources and comparing them to the appearance on plain films, most valves can be identified when this becomes necessary during shunt “troubleshooting.”
“Pumping” the shunt to assess its function involves pressing on the reservoir or valve and assessing how it refills; this requires experience with the specific shunt type and can be interpreted only in the context of the ventricular size or volume of fluid in the space being drained. Tapping the shunt requires identifying the reservoir, which is specifically designed for this purpose. A shunt survey (plain radiographs of the shunt along its course) will usually show its location. The part of the shunt that is “pumped” usually, but not always, is the part that is tapped. For example, with a Holter-type valve, a separate (Rickham) reservoir is usually placed in the burr hole itself (Fig. 42.3); tapping the pumping chamber of the cylindrical Holter valve may cause it to be damaged.
As always, there are exceptions and variations in shunt components. Proximal catheters are sometimes multiple and may drain other cavities such as cysts, a trapped fourth ventricle, or the subdural space. Some shunts do not have reservoirs or valves, and some components may be radiolucent on radiograph. The consulting neurosurgeon can usually help sort out these less common situations.
Indications
Shunt Malfunction
Children with a ventricular shunt malfunction often present with signs and symptoms of increased intracranial pressure, such as headache, vomiting, irritability, and lethargy. Sunsetting (inability to look up) or crossed eyes may be seen, and in infants the fontanel may be full. Occasionally, less common symptoms will herald malfunction, such as seizures or other neurologic symptoms. In severe cases, coma, herniation, arrest, and death can occur (2). Families often recognize and diagnose shunt problems with a high degree of accuracy, especially because each shunt malfunction tends to present with the same signs in a given child. Their opinion is a valuable resource not to be overlooked.
Shunt malfunction may result from obstruction of the proximal catheter (usually with choroid plexus) or the distal system from one of the following: (a) malfunction of a valve; (b) improper setting of a programmable valve due to application of a strong magnetic field, as with magnetic resonance
imaging (MRI); (c) a short shunt due to growth; (d) shunt fracture or disconnection, or (e) distal catheter occlusion. Proximal obstructions, which are the most common cause of shunt malfunction, are diagnosed by a slowly refilling shunt or minimal flow on shunt tap in the presence of ventricular enlargement on computed tomography (CT) scan or MRI. Shunt disconnections or insufficient distal length are diagnosed by shunt survey. Most other cases of shunt malfunction involve distal obstruction or valve malfunction (2).
imaging (MRI); (c) a short shunt due to growth; (d) shunt fracture or disconnection, or (e) distal catheter occlusion. Proximal obstructions, which are the most common cause of shunt malfunction, are diagnosed by a slowly refilling shunt or minimal flow on shunt tap in the presence of ventricular enlargement on computed tomography (CT) scan or MRI. Shunt disconnections or insufficient distal length are diagnosed by shunt survey. Most other cases of shunt malfunction involve distal obstruction or valve malfunction (2).
In general, proximal obstructions cause the most acute neurologic symptoms—and may require urgent surgical correction—because a high-grade proximal obstruction precludes relief of pressure by a shunt puncture. If the problem is elsewhere in the shunt, or if the proximal obstruction is partial, tapping the shunt will usually allow withdrawal of sufficient CSF to relieve the more severe symptoms temporarily until definitive surgical repair can be performed. The CSF pressure and the ease with which fluid can be withdrawn also help evaluate shunt function. If symptoms are mild, and if the diagnosis of shunt malfunction is clear by noninvasive means, temporary medical management with diuretics such as acetazolamide can be helpful until surgical correction is accomplished, as the risks associated with shunt puncture can thus be avoided (Table 42.1). Consequently, not every child with a suspected shunt malfunction will need to undergo a shunt tap.
Shunt Infection
Shunt infections nearly always present with fever, which may be low grade, intermittent, or high grade. Abdominal pain or, uncommonly, frank peritonitis may be seen with infected VP shunts. Redness or tenderness over the shunt may also be apparent. With VA shunts, nephritis is a rare but serious complication of shunt infection. Although a severe shunt infection may lead to obstruction and shunt failure, the majority of infected shunts continue to function, so increased intracranial pressure is not part of the typical picture of early shunt infection (2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree