30 Vaginitis and Vulvitis Natasha R. Johnson Vaginitis is defined as the group of conditions that cause vulvovaginal symptoms such as itching, burning, irritation, and abnormal vaginal discharge. Etiologies of vaginitis can be infectious or noninfectious. The most common causes of infectious vaginitis are bacterial vaginosis (BV), vulvovaginal candidiasis (VVC) and trichomoniasis. Noninfectious etiologies include atrophic vaginitis, allergies, chemical irritation, and various vulvar dermatological conditions. Vaginitis can be associated with sexually transmitted infections, including human immunodeficiency virus (HIV), postsurgical infections, and adverse reproductive outcomes in pregnant women. Vaginitis has a wide differential diagnosis (Table 30.1) and successful treatment relies on accurate diagnosis. Vaginitis is a common gynecologic problem in the general population, accounting for over 10 million office visits per year. Vaginitis can occur in any age-group (prepubertal, reproductive age, menopausal), with the etiology of vaginitis varying according to estrogen status and individual patient risk factors. Three major types of vaginal infection cause up to 90% of vaginitis cases: BV (22–50%), VVC (17–39%), and trichomoniasis (4–35%). In reproductive aged women, physiologic or normal discharge is white or transparent, mostly odorless and pools in the fornices of the vagina. Normal vaginal discharge contains vulvar secretions from sebaceous, sweat, Bartholin and Skene glands, transudate from the vaginal wall, exfoliated vaginal and cervical cells, cervical mucous, endometrial and tubal fluids and normal bacteria and their metabolic products. Normal vaginal discharge typically does not cause symptoms of burning or itching. The quantity and quality of normal vaginal discharge are influenced by the menstrual cycle. The discharge may become more noticeable at times, for example, during pregnancy or at midmenstrual cycle close to the time of ovulation. Age, hormones, sexual activity, contraception, pregnancy, presence of foreign bodies, use of hygienic products, or antibiotics can disrupt the normal fora of the vagina and allow pathogens to grow.
Vaginitis
Prevalence and Epidemiology
Pathophysiology / Normal Vaginal Physiology and Flora
| Condition | Causes |
| Infectious vaginitis | |
| Bacterial vaginosis | Gardnerella vaginalis, Mycoplasma hominis, Mobiluncus species, Bacteroides species (other than Bacteroides fragilis) |
| Vulvovaginal candidiasis | Candida albicans, C. glabrata, C. Tropicalis |
| Trichomoniasis | Trichomonas vaginalis |
| Bacterial vaginitis | Group A Streptococcus |
| Desquamative inflammatory vaginitis | Unknown cause, clindamycin and steroid-responsive |
| Ulcerative vaginitis | Staphylococcus aureus and toxic shock syndrome, HIV-associated |
| Noninfectious vaginitis | |
| Atrophic vaginitis | Estrogen deficiency (menopausal and postpuerperal) |
| Chemical irritation | Soaps, perfumes, hygiene products (tampons, pantyliners), latex condoms |
| Allergic, hyper-sensitivity vaginitis and contact dermatitis (lichen simplex chronicus) | Sperm, douching, dyes, inhaled allergens, occupational exposures, hygiene products (tampons, pantyliners), latex condoms, diaphragms |
| Lichen planus (erosive type) | Flat, hyperkeratotic lesions; pruritic or painful; associated with vulvar and oral lesions |
| Foreign body with or without infection or trauma | Tampons, contraceptive devices, pessary, others |
Source: Modifed from Owen MK, Clenney TL. Management of vaginitis. Am Fam Physician 2004;70:2125-2132 and Sobel JD Vaginitis N Engl J Med 1997;337:1896-1903
Lactobacilli (Gram-positive rods) are the predominant bacteria in the vagina and the regulator of normal vaginal fora. Lactobacilli make lactic acid and hydrogen peroxide which maintains the normal vaginal pH of 3.8–4.5. This acidic environment inhibits the adherence of bacteria to vaginal epithelium and prevents the growth of pathogens. Estrogen status plays a critical role in determining the normal state of the vagina by enhancing lactobacilli colonization. In prepubertal girls and postmenopausal women who have low levels of estrogen, the vaginal epithelium is thin and the pH of the vaginal secretions is 4.7 or higher. The more basic pH is due to the reduced quantity of lactobacilli. Although lactobacilli are the dominant bacteria in the vagina, other bacteria are also present, such as streptococcal species, Gardnerella vaginalis, Gram-negative bacteria and anaerobes. Candida albicans can also be found in normal fora in 10–25% of asymptomatic women. Thus, a routine bacterial culture will demonstrate a broad variety of organisms including skin and fecal flora.
Acute vaginitis due to BV reflects a disruption in the normal ecosystem of the vagina from lactobacilli-dominant to mixed fora including Gardnerella vaginalis, Mycoplasma and anaerobes such as Peptostreptococci, Pre-votella and Mobiluncus species. VVC and trichomoniasis result from an overgrowth of candidal species and infection with Trichomonas vaginalis, respectively.
Diagnosis
Both the history and physical examination findings are relatively nonspecific. Symptoms such as pruritis and the characteristics of the discharge (e.g., amount and color) do not reliably predict the cause of the acute vaginitis. However, certain features suggest a particular acute vaginitis diagnosis (Table 30.2), which can then be confirmed by office analysis (Table 30.3). Women frequently diagnose and treat their vulvovaginal symptoms, especially for presumed VVC, with over-the-counter preparations, which may be inappropiate due to inaccurate self-diagnosis. In one study, 95 women who self-diagnosed VVC and purchased over-the-counter antifungal therapy were evaluated and only 34% were found to have VVC on final diagnosis. Although misdiagnosis may have minimal effects on vulvovaginal symptoms, such as itching, odor, or discharge, it may be of greater concern if a patient who self-treats for VVC actually has pelvic inflammatory disease or a urinary tract infection. While tempting to treat empirically, based on symptoms alone, studies have shown poor correlation between symptoms and final diagnosis, so this practice should be avoided.
| Step 1 | History and physical examination |
| Step 2 | Vaginal pH determination |
| Step 3 | Saline and potassium hydroxide microscopy |
| Step 4 | “Whif” test |
| Step 5 | Vaginal culture for yeast, rapid diagnostic test or culture for Trichomonas if microscopy negative |
| Cervical culture or PCR for Chlamydia and Gonorrhea if clinically indicated |
History
Evaluation of women with vaginitis should include a focused history about the vaginal symptoms, including characteristics of the vaginal discharge (color, consistency, and malodor), itching, burning, irritation, swelling, dyspareunia, and dysuria. Directed questions about location of symptoms (vulva, vagina, anus), duration, relation to the menstrual cycle, response to prior treatment including self-treatment with over-the-counter or prescription medications, and recent antibiotic use are important to help determine the etiology. Review of hygienic practices (douching, soaps, detergents, perfumes, powders, sprays, lubricants, daily use of pantyliners) may elicit potential exposure to irritants and allergens and habits detrimental to vulvar skin. Obtaining a sexual history is important as exposure to a new sexual partner increases the risk of acquiring a sexually transmitted infection including Trichomonas or cervicitis related to Chlamydia trachomatis and/or Neisseria gonorrhoeae. The presence of any associated symptoms should also be ascertained. For example, the presence of associated abdominal/pelvic pain is suggestive of pelvic inflammatory disease and supra-pubic pain (especially with dysuria) may indicate cystitis, both uncommon in vaginitis.
Physical Examination
The physical examination should begin with careful evaluation of the vulva, as many patients with vaginitis have vulvar symptoms. Signs of erythema, thickening, fissures, excoriations, plaques, ulcers, thinning, and discharge at the introitus should be noted and can give clues to the etiology. In almost all patients, further assessment will include a speculum examination to inspect the vagina (noting lesions, erythema and characteristics of any discharge present), the cervix (noting lesions, erythema and “mucopus” or purulent cervical mucous) and to collect specimens for office analysis and microbiology testing. Cervical inflammation is suggestive of cervicitis, not vaginitis, and the cervix typically is erythematous, friable, and with a mucopurulent discharge. A bimanual and abdominal examination should be performed to elicit pelvic and cervical motion tenderness if the patient has abdominal and/or pelvic pain and there is concern for pelvic inflammatory disease.
Office Analysis
Simple office analysis including pH testing, amine-”whif” test, saline (wet mount) and potassium hydroxide (KOH) microscopy and vaginal/cervical cultures when indicated are important tools in the diagnosis of vaginitis. These tests can be performed by obtaining samples of the vaginal discharge during speculum examination. Vaginal pH should be measured by touching a cotton-tipped swab to the sidewall of the vagina and touching the swab directly to commercially available pH paper (expanded in the pH range 4.0–5.5). The samples should be taken from the lateral vaginal fornix (midway between the introitus and cervix) to avoid false elevations in pH results caused by cervical mucus, blood, semen, or other substances that pool in the posterior fornix. Measurement of vaginal pH is the single most important finding in the diagnostic process and should always be determined. A pH above 4.5 in a premenopausal woman suggests BV or trichomoniasis and can help exclude VVC which typically has a pH in the 4–4.5 range. Premenarchal and postmenopausal women typically have a vaginal pH of 4.7 or higher and pH measurement in these groups is less useful in the evaluation of vaginitis symptoms.
Vaginal discharge can be examined by preparation of a saline and potassium hydroxide (KOH) wet mount. A sample of vaginal discharge is taken from the lateral vaginal fornix using a cotton-tipped swab and placed in one or two drops of 0.9% normal saline solution on one slide and a second sample in 10% KOH solution. Cover slips are placed on the slides and they are examined under a microscope at low and high power. A normal microscopic examination will reveal a predominance of squamous cells and rare polymorphonuclear leukocytes. Clue cells (epithelial cells with borders studded by small bacteria, characteristic of BV), white blood cells, lactobacilli and motile trichomonads are usually identified in the saline specimen. Hyphae or yeast buds of candidal species are more easily seen in the KOH specimen, as KOH lyses cellular elements. An amine odor (“fishy smell”) detected immediately after adding 10% KOH to the vaginal discharge sample on the slide is called a positive “whif” test and suggests BV.
Initial office evaluation (history and physical examination, vaginal pH, wet mount, microscopy, and “whif” test) has been shown to correctly diagnose 60% of candidal vaginitis, 70% of trichomonad vaginitis, and 90% of BV. In patients with an initial nondiagnostic evaluation, vaginal cultures for yeast and rapid diagnostic tests or culture for Trichomonas (if pH >4.5 and suggestive clinical symptoms) should be performed. If the evaluation excludes the three most common causes of vaginitis (VVC, BV, trichomoniasis), then other causes of vaginitis need to be considered (see Table 30.1). Patients considered at risk for sexually transmitted infections—with purulent vaginal or cervical discharge, fever or lower abdominal / pelvic pain—should have a cervical sample obtained for Chlamydia trachomatis and Neisseria gonorrhoeae testing.
Types of Vaginitis
Vulvovaginal Candidiasis
Approximately one-third of acute vaginitis is due to candidal vulvovaginitis. Most cases of acute VVC are caused by Candida albicans, but occasionally are caused by other Candida species such as C. glabrata and C. tropicalis. VVC is common in adults, approximately 75% of women will receive a diagnosis of VVC at least once, and of those, about 50% will have a recurrence. Candidal species can be found in normal fora in 10–25% of healthy asymptomatic women and likely access the vagina via migration from the rectum across the perineum. Symptomatic infection is correlated with a high vaginal fungal load and vaginal infiltration of polymorphonuclear neutrophils. The mechanism by which Candida species overgrow and cause symptomatic disease is multifaceted, involving host inflammatory response to invasion and yeast virulence factors. Risk factors for symptomatic infection include antibiotics (inhibit normal bacterial fora), high-estrogen contraceptives (but not low-dose oral contraceptives), pregnancy, diabetes mellitus with poor glycemic control, immunosuppression (e.g., HIV, patients taking corticosteroids), and contraceptive devices (vaginal sponges, diaphragms, intrauterine devices). VVC is not traditionally considered a sexually transmitted disease as it occurs in nonsexually active women and because Candida is considered part of the normal vaginal fora. However, sexual transmission of Candida can occur and VVC is associated with sexual activity. Evidence for this include: 1) an increase in frequency of VVC at the time most women begin regular sexual activity; 2) partners of women infected with VVC are more likely to be colonized with Candida than partners of uninfected women; and 3) VVC episodes may be linked to orogenital and anogenital sex.
Clinical features and diagnosis: Typical symptoms of VVC include vulvar pruritis, burning, vulvar and vaginal soreness, irritation, dysuria (external) and a thick, white vaginal discharge. Physical examination may reveal vulvar erythema, edema, fissure formation and a thick, white, adherent, “cottage cheese-like” vaginal discharge (Fig. 30.1).
Vaginal pH of less than 4.5 and a negative “whif” test can help distinguish VVC from BV and trichomoniasis. However, diagnosis requires either (1) visualization of blastospores or pseudohyphae on saline or 10% KOH microscopy (Fig. 30.2), or (2) a positive culture for yeast in a symptomatic woman. A culture for Candida is indicated by clinical findings if microscopy is negative because microscopy is not sufficiently sensitive to exclude these diagnoses in symptomatic patients. Studies have shown that microscopy can be negative in up to 50% of patients with confirmed VVC. Routine culture is not warranted as an initial diagnostic test for all patients because of its cost, the delay in timely diagnosis, and that it may be positive due to asymptomatic colonization.
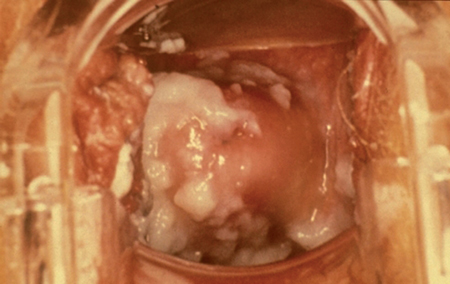
Fig. 30.1 Speculum examination showing characteristic vaginal discharge of vulvovaginal candidiasis is thick, white, curdy, and “cottage cheese”-like. Printed with permission from Seattle STD/ HIV Prevention Training Center, Washington State, Department of Health.
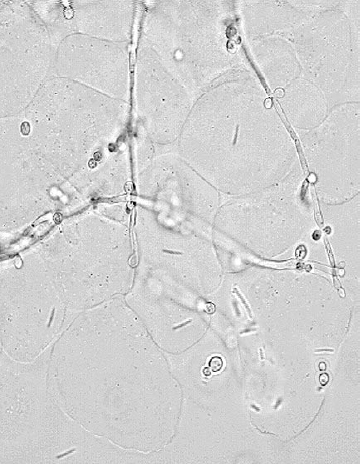
Fig. 30.2 KOH microscopy of candidiasis reveals yeast pseudohy-phae, budding yeast and lysed epithelial cells Printed with permission from Seattle STD/HIV Prevention Training Center, Washington State, Department of Health.
Recurrent VVC (four or more documented episodes in one year) occurs in less than 5% of the population. Culture should be performed in women with persistent or recurrent symptoms to confirm the diagnosis, and because many of these women may have non-albicans infections that are resistant to standard therapy. Predisposing factors are identifable in only a minority of women including diabetics with poor glycemic control and those undergoing immunosuppressive therapy. Other factors that predispose to recurrent VVC are poorly understood and include abnormalities in local vaginal mucosal immunity and genetic susceptibility which is mediated through specific gene polymorphisms.
Treatment:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


