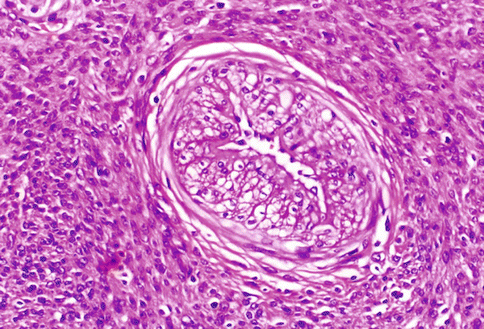
Fig. 3.1
A typical uterine leiomyoma, composed by bundles of spindle cells, similar to those present in the myometrial wall: only the whirling and irregular arrangement of the cellular bundles distinguish the neoplastic proliferation. The large and ribbon-like eosinophilic cytoplasm is evident also at low magnification. In the present picture, a small amount of hyaline connective tissue is organized around the arterial vessels and among the cellular fascicles
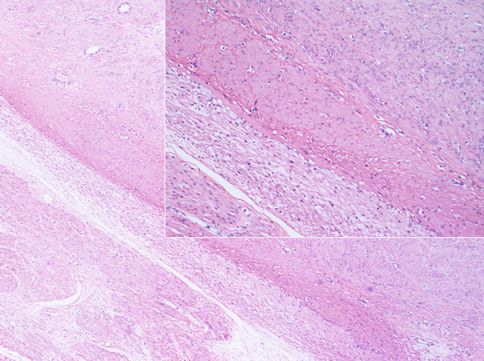
Fig. 3.2
Most of benign uterine leiomyomas shows defined margins as respect to the normal myometrium. The compression of normal smooth cells around the tumor forms a “pseudocapsule” from which the surgeon delivers the mass during the myomectomy. In insert image, a more accurate observation of the tumoral margins puts in evidence a layer of small compressed native smooth cells. The nuclei of these cells are not regressive: we can conclude that the “pseudocapsule” is an active form of contrast against the tumoral growth
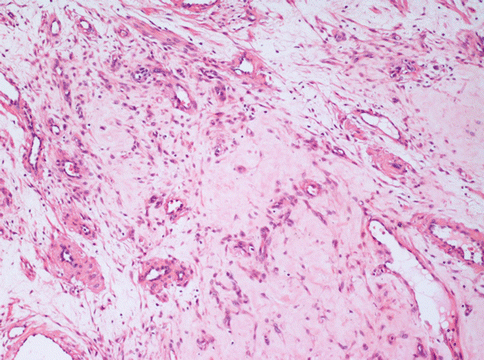
Fig. 3.3
A leiomyoma with a diffuse interstitial oedema. In this area, an otherwise typical uterine leiomyoma, shows few neoplastic cells merged with a large amount of loose interstitium and several vessels. This pattern is different from that of the more dangerous myxoid leiomyioma
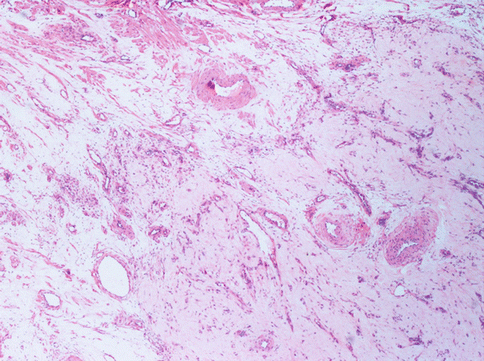
Fig. 3.4
The present uterine leiomyoma is provided of a great amount of vessels: arteries with thick muscular wall, large veins and capillary vessels. The stroma is very oedematous. The special conformation of the tumour (angioleiomyoma) may evocate different diagnosis with US or other instrumental examination
Leiomyomata variants have differences that can be macroscopic or histological and thereby pose a serious problem of differential diagnosis with leiomyosarcoma.
First of all, there can be “infarct-type” necrosis or hyaline necrosis according to Bell et al. [4], characterized by areas of seemingly coagulative necrosis separated from the rest of the tumour by rings of fibrous or granulation tissue (Fig. 3.5). This type of necrosis must not be confused with “tumour cell necrosis” observed in leiomyosarcoma where the cells are pervasively necrotic, there is no separation from the surrounding tumour and often there are clumps of the residue of neoplastic cells surrounding residual vascular structures, commonly known as ghost outlines (Fig. 3.6).
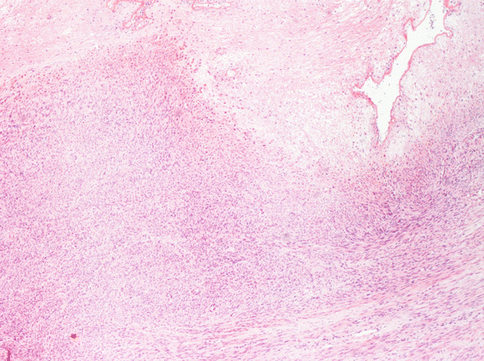
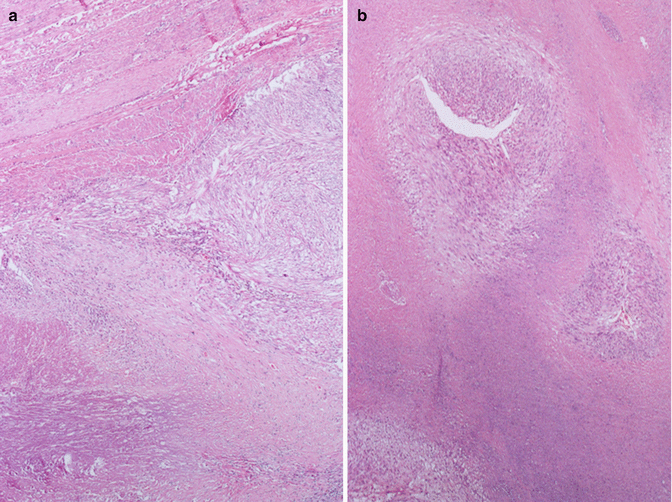

Fig. 3.5
A typical degenerative necrosis in an otherwise typical leyomioma. The regressive hypocellular area (bottom) is sharply limited from the neoplastic vital cells. No granulocyte infiltration is observed at the border of necrosis

Fig. 3.6
In leiomyosarcoma is often present a particular form of necrosis named “tumoral necrosis”. In this figure the necrosis is present in the lower part and it is separate from the vital cells by a layer of granulation tissue characterized by small vessels and infiltration of neutrophil granulocytes. Note the absence of the “pseudocapsule” between the tumor and the normal myometrium (on the bottom) (a). In a “tumoral necrosis” of a leiomyosarcoma the residual vital malignant cells are arranged as cellular poorly defined nodules around two arteries (b)
The differential diagnosis with leiomyosarcoma supposes, other than “tumour cell necrosis”, the presence of moderate-severe atypia and a mitotic index over 10/10HPF (Fig. 3.7): according to the Bell criteria of Bell et al. [4] the presence of two out of three of these paramenters is enough to diagnose leiomyosarcoma. Immunohistochemistry does not bring any further advantages to the differential diagnosis. The borders of the tumour often infiltrate the near myometrium without the presence of the pseudocapsule observed in benign leiomyomata (Fig. 3.8). Clearly malignant tumours express more p16, p21 and p53, but this is not helpful in single cases [5].
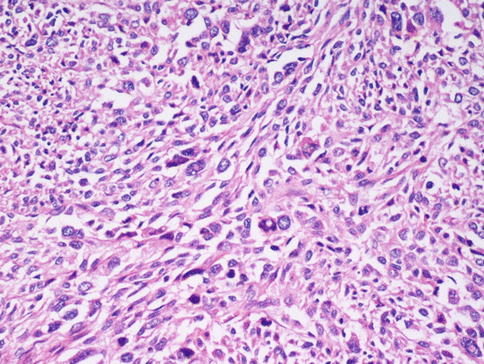
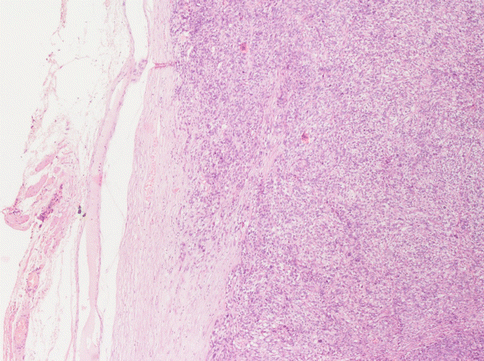

Fig. 3.7
In a malignant smooth muscle cell tumour of the uterus the cells maintain the spindle shape, but the nuclei are dysmetric, hyperchromatic, with rare evident eosinophilic inclusions. Mitoses, also of atypical aspect, are frequent

Fig. 3.8
At the margins of a leiomyosarcoma the “pseudocapsule” is always absent. The neoplastic cells invade the normal myometrium. In this case the peritoneal surface of the uterus (on the left) is obscured by a fibrous scar
The expression of Ki 67 is also higher in leiomyosarcomas: in our experience Ki 67 is constantly higher than 40 %, while in leiomyomata it is always less than 10 %.
Necrosis can also be observed beneath the endometrium in tumours that grow into the uterine cavity which ulcerate and bleed, and therefore the necrotic cells are found together with inflammatory infiltrates.
Other degenerative changes, more of less common, are: hyaline transformation of the stroma (Fig. 3.9), leading to the wide substitution of the tumour, dystrophic calcification for a calcium salt deposition in the interstitial stroma (more common in menopausal women) (Fig. 3.10), a hydropic degeneration that can lead even to the formation of pseudocysts full of clear liquid.
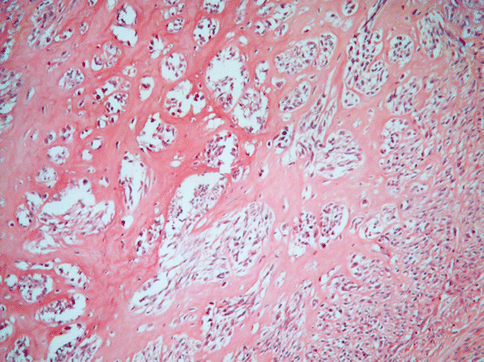
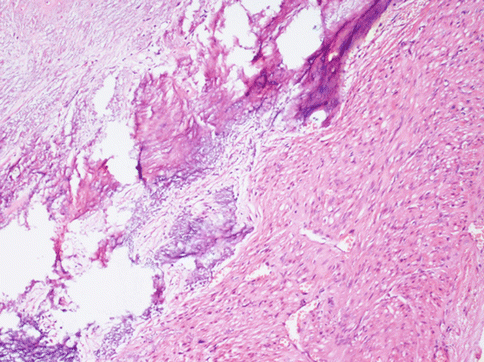

Fig. 3.9
A large part of the stroma in this usual leiomyoma is substituted by hyaline collagen. The neoplastic cells seem to be atrophic and compressed in small clusters

Fig. 3.10
Area of a typical leiomyoma with degenerative hyaline stroma with a subsequent dystrophic calcification and osseous metaplasia. This phenomenon may be focal or involving almost all the neoplasia, especially in perimenopausal women. Such tumours are evident at radiological examination
A rarer but important type of change is myxoid degeneration (Fig. 3.11) of the fibroid when a gelatinous material is formed and the leiomuscolar cells can appear star-shaped and immersed in a slightly basophile stroma. Here the margins of the tumour must be closely examined as well circumscribed and the mitotic index is an essential point. A myxoid leiomyosarcoma, besides invading the surrounding miometrium, displays evident mitotic figures with only 2 per 10 HPF necessary to presume a malignant behaviour.
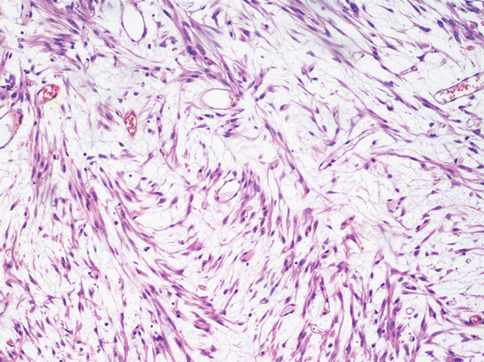

Fig. 3.11
In a myxoid leiomyoma the tumoral spindle cell are dispersed in a basophilic stroma. Several cells assume a stellate form. The diagnosis of this tumour is clinically relevant because the presence of a few mitoses suggests an aggressive behaviour
During pregnancy, leiomyomata can undergo necrosis and suffer bleeding (red degeneration), depending on the hormonal situation. Similar degeneration has been seen in hormonal therapy with estroprogestins. GnRH – analogue therapy causes a high degree of cellularity of the tumour which can confuse the pathologist when analyzing the mitotic figures. The increase in cellularity is apparently an effect linked to cell atrophy which reduces the cytoplasm thickness with closer nuclei, often with densely packed chromatin. As we discussed in a previous paper, the arteries we have described alterations of the inner wall (Fig. 3.12) coherent with the proliferation of muscle cells and distortion or restriction of the lumen [6].