Fig. 13.1
Laparoscopic port placement for robot-assisted myomectomy: standard protocol (pictures a, a1). The camera port is umbilical. Robotic arm ports 1 and 2 are between 8 and 10 cm to the left and to the right of the umbilicus. The assistant port is medial to the anterior-superior iliac spine (either left or right side). It is possible to forgo the assistant port as the team’s experience increases: needles can be inserted through the camera port and suction can be provided through a robotic port without any need for undocking. Robotic arm port 3 should be used for more complex cases: it is placed contra-laterally to the assistant’s port. This entire configuration is shifted cephalad to address larger uterine masses. Laparoscopic port placement for robot-assisted myomectomy: minimal access protocol (pictures b, b1). This is an advanced protocol: no scars are visible above the anterior-superior iliac spines, however the robotic instruments enter the pelvic at a wider angle from the midline and are more difficult to handle. The camera port is umbilical. Robotic arm ports 1 and 2 are medial to the anterior-superior iliac spines. There is no room for robotic arm port 3. The assistant port is suprapubic (or absent, see considerations for standard protocol)
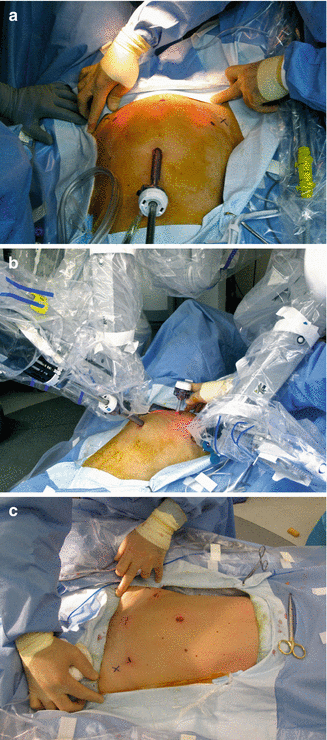
Fig. 13.2
Actual surgical images of patient operated with a minimal access (cosmetic) protocol. (a) Planning of port sites with distended abdomen. Note that port sites are slightly cephalad to the anterior-superiod iliac spines to avoid injury of the ileoinguinal and ileohypogastric nerves. (b) Ample space is created in the suprapubic area for the assistant to use the suprapubic access. (c) Final postoperative effect
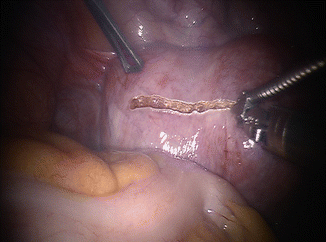
Fig. 13.3
Hysterotomy. This can be performed in a transverse, sagittal or elliptical fashion, depending on myoma location and size and surgeon’s preference. In this particular case a flexible CO2 laser delivery system (BeamPath™, OmniGuide, Cambridge, MA) connected to a robotic needle driver is employed. This setup allows full use of the wristing ability of the robotic instruments. Robotic Harmonic sheers are an excellent alternative and also provide minimal thermal spread and therefore minimal myometrial damage. A drawback of the robotic Harmonic sheers is that the long vibrating element does not allow any movement of the wrist, thereby limiting the ergonomic advantage of the robotic platform. Use of robotic monopolar sheers, albeit very popular, is the least appropriate for reproductive surgery applications, including myomectomy. If robotic monopolar shears are employed, one should do so with exclusive cutting – not coagulating – current settings
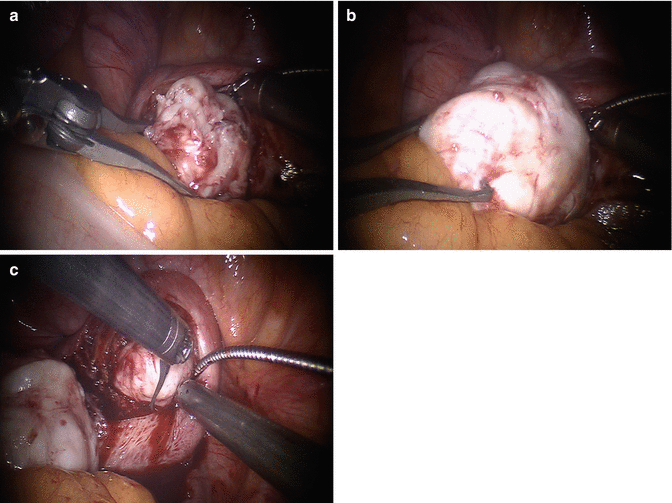
Fig. 13.4
Myoma enucleation. The goals of myoma enucleation are: (1) reduce the number of hysterotomies needed for enucleation of all myomas, and (2) avoid injury to the endometrial cavity and the tubal lumen. Once the space between the pseudocapsule and the tumor is entered with use of energy, dissection continues in a blunt fashion for most of the myoma enucleation. The robotic tenaculum is not designed to pull the myoma out of its pseudocapsule: rather it is used to steady the tumor while other instruments make progress in detaching the pseudocapsule from it. Some areas of the pseudocapsule are tightly connected to the myoma and may require focal energy use, rather than blunt dissection, to complete the least traumatic enucleation. We perform chromopertubation in advance of any myomectomy where breach of the endometrial cavity is a possibility. Chromopertubation is not done to assess tubal patency, as this has minimal relevance and has little predictive value on future tubal function, but to stain the endometrial cavity and make it easy to identify an entry. (a) Robotic tenaculum stabilizes the myoma and instrument 1 opposes counter-traction to facilitate enucleation. (b) Enucleation almost complete. (c) A second myoma is removed through the same incision: economy of incisions is a key factor in every myomectomy
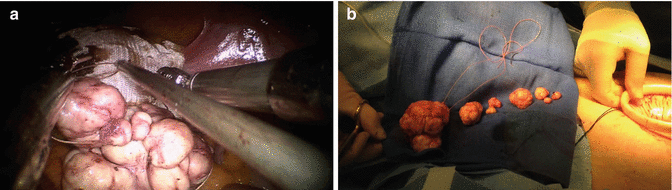
Fig. 13.5
Myoma string. In cases where many small myomas are enucleated, we keep a written myoma count at bedside and we collect those myomata that could be lost in the abdomen on a suture line. A barbed suture is particularly helpful for this task, as myomas can be made to slide towards the loop end of the suture and cannot slide back. A suture life-line is also helpful to keep the myomas collected for later placement in a containment system (see Fig. 13.7 below). (a) Several small myomas on a string (coaxial single-incision robot-assisted myomectomy). (b) Same patient with myomata recovered through single umbilical incision
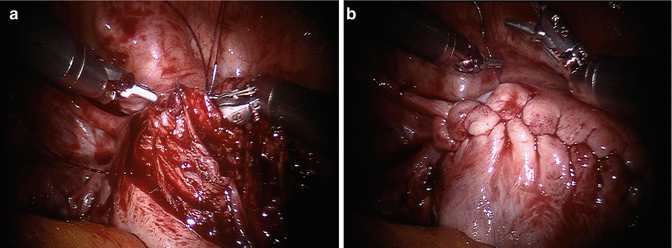
Fig. 13.6
Repair of hysterotomy in layers. The goals of hysterotomy closure are: (1) anatomically correct reapproximation of all layers, (2) mechanical control of hemostasis and avoidance of hematoma formation, and (3) avoidance of exposed suture, exposed myometrium and coagulated serosa (highly adhesiogenic foci). The suture of choice for myomectomy is barbed suture. Deep layers are closed in a simple running fashion and the final layer (perimetrium ad serosa together) is closed with a running non-locking imbricating “baseball stitch” suture. A well performed baseball stitch will have no barbed suture visible and constitutes a safe and highly hemostatic closure. Occasionally, we employ 3.0 or 2.0 non-barbed suture to reapproximate an endometrial laceration or a small superficial hysterotomy. (a) Closure in several layers is essential to avoid hematoma formation. (b) Baseball stitch demonstrating no visible barbed suture
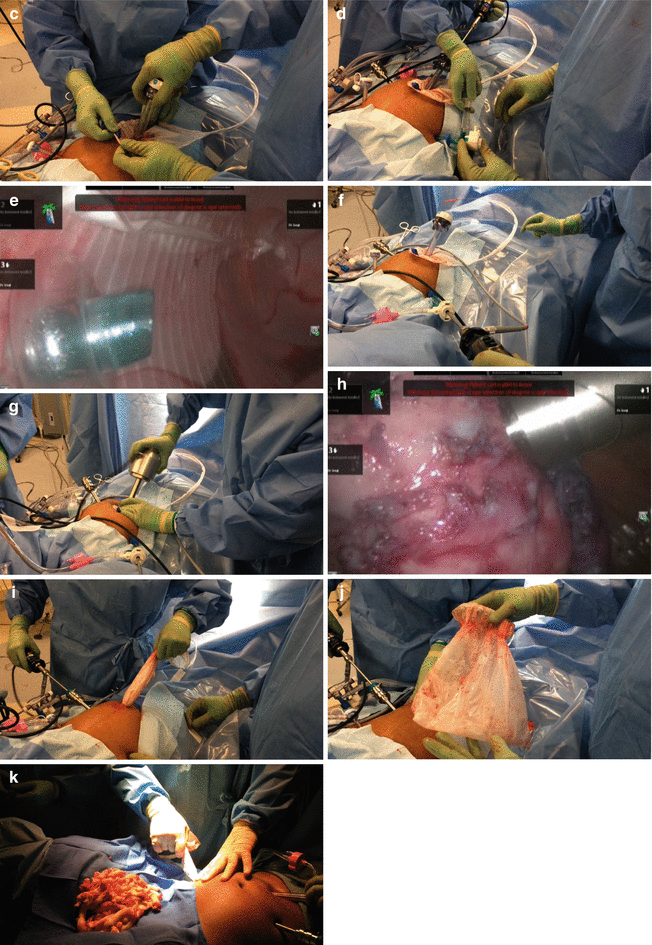
Fig. 13.7




Morcellation. This step is not performed with robotic assistance but rather with conventional technique. Electromechanical morcellation of myomas in the abdominal cavity remains a viable option for myoma extraction following minimally invasive myomectomy, provided patients are duly informed of the underlying risk of unintended morcellation of a malignant tumor. In those cases where the benign histologic nature of the myoma is in doubt, morcellation in a containment system should be considered to virtually eliminate the risk of tumor dispersion in the abdominal cavity. Electromechanical laparoscopic morcellation can be safely performed within a containment system. (a) Laparoscopic sac is placed through a 15 mm umbilical port. (b) After myomas are placed in the bag, the 15 mm port is removed. (c) The 15 mm port is re-inserted, this time inside the laparoscopic sac; the sac is insufflated. (d) A 5 mm camera port is inserted in place of one of the robotic instrument ports; the camera port pierces the sac and its pneumatic fixation tip is insufflated. (e) The pneumatic fixation tip of the 5 mm port is insufflated and in place within the insufflated specimen sac. (f) The laparoscope is now inserted through the 5 mm port, so that the 15 mm port can be removed again. (g) The morcellator is set in place of the 15 mm port. (h) Morcellation proceeds in a completely contained system. (i–j) The laparoscopic sac is removed and examined to confirm that it is intact. (k) An identical technique can be used for morcellation through a lower quadrant port if preferred
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


