Fig. 15.1
Oral contraceptives do not modify the risk of myomas and do not influence their growth
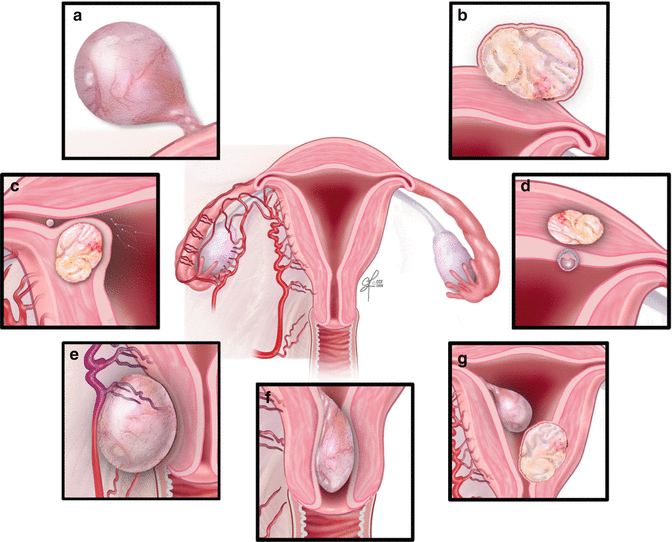
Fig. 15.2
Classification of fibroids; (a) – pedunculated; (b) – subserosal; (c) – submucosal; (d) – Intramural; (e) – within the broad ligament; (f) – cervical; (g) – intracavitary
Myomas are highly sensitive to, and dependent on, estrogen and progesterone. This is reflected in the prevalence during reproductive years and regression after menopause (Fig. 15.3) with cessation of ovarian activity. Myoma tissue maintains an elevated concentration of estrogen, from ovarian production and from local conversion by abundant aromatase within myoma cells. Progesterone is responsible for cell proliferation and cell growth. Activation of estrogen receptor α (alpha), present in myoma tissue, allows and enhances tissue response to progesterone [2].

Fig. 15.3
Myomas generally undergo a volumetric regression after menopause, as in post-menopausal patients needing transdermal hormonal replacement therapy
With the increasing number of women pursuing childbearing later in life, improved ultrasound technology and widespread use of prenatal ultrasound, there has also been an increase in documented fibroids during pregnancy [4]. It has been reported that fibroids are present in 1.6–4.7 % of pregnancies [5].
Fibroids and Infertility
Pain, bleeding and bulk symptoms have been proven attributable to fibroids (Fig. 15.4), however, the impact of fibroids on fertility has been difficult to establish. As stated, it is estimated that fibroids are present in 20–40 % of reproductive aged women, but have only been identified in 5–10 % of infertile women [6]. Studying the relationship of fibroids to fertility is further complicated by the heterogeneous nature of fibroids including size, location, composition and number.
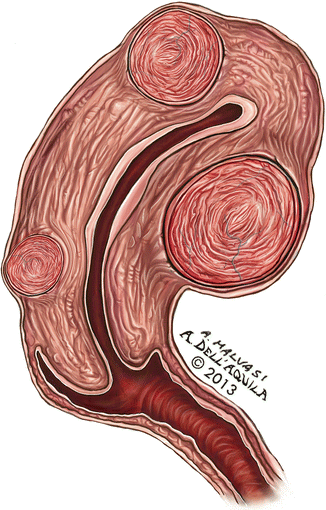

Fig. 15.4
The uterine myomas may cause pain, bleeding and bulk symptoms
When considering the impact a fibroid may have on fertility, location is of utmost importance. Proposed mechanisms based on location include fibroid displacement of the cervix impacting sperm exposure, deformity of the uterine cavity interfering with implantation and gamete transport, obstruction of the tubal ostea, distortion of adnexal anatomy, impaired contractility of surrounding myometrium altering sperm or embryo transport and altered endometrium overlying fibroids impairing implantation [7]. Common themes among these proposed mechanisms include decreased fertility due to impaired sperm or oocyte transport and decreased embryo implantation. Based on this notion, it makes sense that the closer the fibroids are to the endometrial cavity, the greater the impact on fertility (Fig. 15.5).
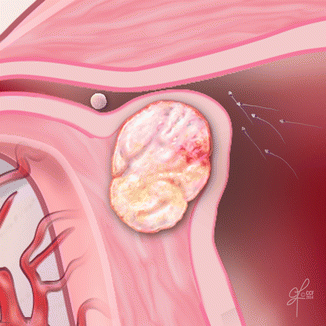

Fig. 15.5
Submuscosal fibroid blocking tubal ostea and/or impairing oocyte and sperm transport
This is evident in the literature. The majority of studies evaluating the effect of fibroids on fertility has focused on reproductive outcomes of patients with unexplained infertility before and after myomectomy or the presence of fibroids in women undergoing IVF cycles since these settings can best control for confounding factors. In both of these instances, there is resounding data that submucosal fibroids negatively impact clinical pregnancy (OR = 0.3, CI = 0.1–0.7) and delivery rates (OR = 0.3, CI = 0.1–0.8) [7–12], and are attributable to a three times increased risk of miscarriage [10, 11]. The relationship of intramural fibroids and infertility is less defined. Studies evaluating IVF outcomes in women with and without intramural fibroids have been inconsistent. Some studies, including a 2005 systematic review evaluating six studies, found intramural fibroids were attributable to significantly decreased implantation (OR = 0.62, CI = 0.48–0.8) and live birth rates (OR = 0.69, CI = 0.5–0.95) [8]. This was supported by a 2007 meta-analysis of seven studies again showing a decreased pregnancy (OR = 0.8, CI = 0.6–0.9) and delivery rate (OR = 0.7, CI = 0.5–0.8) [9]. Twenty-three studies compiled in a 2009 systematic review of the literature on the topic found that fertility outcomes were decreased in women with intramural fibroids including a decreased clinical pregnancy rate (RR 0.8, CI 0.7–0.94) and increased rate of spontaneous abortion (RR 1.75, CI 1.23–2.49) [11]. Alternatively, other studies have failed to show a significant effect of intramural fibroids on IVF outcomes [10, 13–18]. Data concerning subserosal fibroids and fertility is more consistent showing no evidence of significant deleterious effects on clinical pregnancy and implantation rates and no increased risk of miscarriage [11].
In conclusion in regard to location, there is evidence that intracavitary and submucosal fibroids may have a negative effect on IVF success and miscarriage rates, that data on intramural fibroids are controversial and that subserosal fibroids likely have no adverse impact on fertility. However, the size and number of fibroids must be considered with larger size and multiple myomas having a more significant effect on fertility, regardless of location [13, 19, 20].
Just as there is not a clear cut consensus on the effect of fibroids on fertility, treatment of fibroids to improve fertility is controversial. Medical management of fibroids is often counterproductive to conceiving and surgical treatment can incur risks both to the health of the patient and for future fertility. The most straightforward circumstance involves the submucosal fibroid with clear distortion of the uterine cavity. The treatment of submucosal fibroids can generally be achieved by hysteroscopic myomectomy (Fig. 15.6). This has lower risks than intra-abdominal surgery, but has the significant risk of developing scar tissue within the uterus that can impede future fertility. The previously mentioned 2009 systematic review showed a twofold increase in clinical pregnancy rates in IVF after myomectomy of submucosal fibroids compared to women without fibroids [11]. There have also been randomized trials comparing expectant management of fibroids compared to treatment with myomectomy with a significant increase in pregnancy rates among patients with submucosal fibroids [21]. When weighing the risks and benefits of surgical management of cavity-distorting submucosal fibroids in regards to fertility, it seems prudent to offer myomectomy to improve reproductive outcomes.
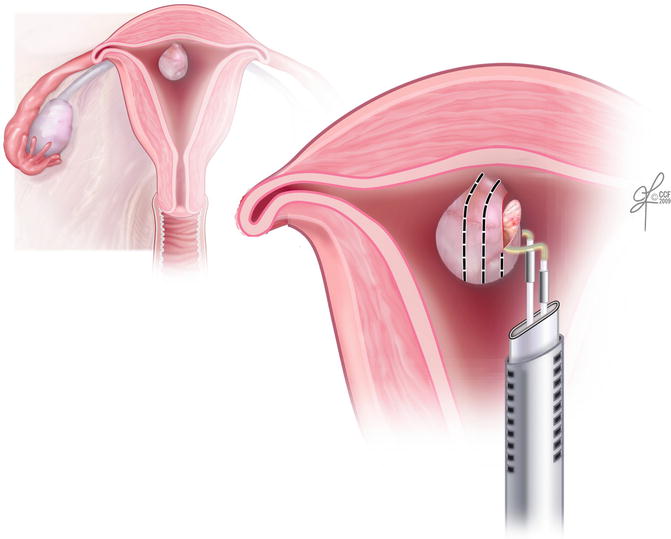

Fig. 15.6
Hysteroscopic resection of an intracavitary fibroid
The treatment of intramural fibroids to improve fertility outcomes is debated as their effect on fertility is less clear. Again, size and number play a role in determining the beneficial effects of surgical treatment. Small, single intramural fibroids have less of an effect on fertility; therefore surgical risks may outweigh the benefits in these patients. Alternatively, there was a cohort study showing that in women with at least one intramural fibroid greater than 5 cm in size, myomectomy significantly improved clinical pregnancy and live birth rates after up to three IVF cycles [22]. However, there are also studies with contradicting results, showing no benefits of myomectomy of intramural fibroids in asymptomatic infertile couples. It appears that in these patients the duration of infertility and other confounding fertility factors have a greater effect on fertility outcomes rather than the size or location of the largest fibroid surgically removed [23, 24].
It is in the management of these patients, infertile women with asymptomatic intramural fibroids, that it is essential that surgical complications must be carefully considered. Since it has not been concretely established that intramural fibroids have a deleterious effect on fertility, and surgical complications such as pelvic and adnexal adhesions, intra-abdominal infections and intrauterine scarring can negatively affect fertility, surgical management must be thoughtfully considered. Age of the patient, infertility history and planned fertility treatments contribute to this decision, as well as size, location and number of the fibroids. Large intramural fibroids that distort the uterine cavity may have the same negative effect on fertility as submucosal fibroids, while smaller fibroids with no endometrial involvement likely do not. Adhesion formation will also be affected by location of myomectomy, with cornual or posterior fibroids of more concern than fundal or uterine body ones.
While the surgical removal of submucosal fibroids by hysteroscopic myomectomy will likely improve fertility outcomes, intramural myomectomies need to be carefully considered and intra-abdominal removal of subserosal fibroids likely pose no benefit to infertile women. In addition, asymptomatic women with untested fertility may want to attempt conception prior to surgical management of any fibroids as they may not warrant any treatment.
Regardless of fibroid location, when attempting successful surgical management for fertility purposes, it is imperative to use meticulous surgical technique to decrease complications and lead to the best surgical and fertility related outcomes. Careful tissue handling, adequate hemostasis, less exposed suture and minimal tissue trauma can lead to improved results. When fibroids are completely or mostly submucosal in location, myomectomy can be performed hysteroscopiclly. Intramural fibroids require intra-abdominal entry which can be obtained in one of three ways. Open surgery by mid-line, pfannenstiel or ‘minilaparotomy’ incision has been a mainstay of gynecologic surgical technique. Myomectomy can also be performed by laparoscopic and/or robotic methods with similar final outcomes with the advantage of decreased blood loss, decreased postoperative pain, shorter hospitalizations and shorter overall recovery time [25–28] (Fig. 15.7). In 2007, there was a multicenter, randomized trial looking at reproductive outcomes after minilaparotomy and laparoscopic myomectomy that found no significant differences in clinical pregnancy, live birth and spontaneous abortion rates between the two surgeries in women with otherwise unexplained infertility [26].
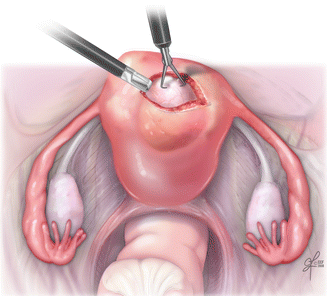

Fig. 15.7
Myomectomy by minimally invasive method
In conclusion, submucosal fibroids likely have a significant effect on fertility and patients can potentially benefit from generally low risk hysteroscopic surgical removal. Intramural fibroids, however, may have a negative impact on fertility, but this depends on their location, size and number and surgical removal can be considered in patients with long-standing otherwise unexplained infertility as long as surgical risks and benefits are thoughtfully compared. Subserosal fibroids likely have no effect on fertility and only should be removed if the patient is otherwise symptomatic. Each patient must be uniquely considered when evaluating for surgical management of fibroids to improve fertility outcomes and be aware of the risk-benefit profile.
Diagnosis and Monitoring of Fibroids in Pregnancy
The majority of fibroids in pregnancy are diagnosed incidentally on routine obstetric ultrasounds as it is been reported that during pregnancy, clinical exam can only detect 42 % of fibroids >5 cm, and that drops to a dismal 12.5 % when they are <5 cm [29]. When a mass is suspected, ultrasound is non-invasive, relatively inexpensive, fast, widely available and safe in pregnancy making it the first line imaging technique. In skilled hands, ultrasound can predict the nature of a pelvic mass with over 80 % accuracy, and can provide helpful information such as size, shape, consistency and location [29].
MRI is another imaging modality that adds value during pregnancy, allowing for a more precise and reproducible mapping of fibroid location and size. This is particularly helpful when there is diagnostic inconsistencies or for use in surgical planning. Unfortunately, the use of MRI is limited by availability, expense and need for experienced observers [30].
When detected incidentally, fibroids only need to be followed by serial imaging if there is concern for fetal development, utero-placental insufficiency due to fibroid location in close proximity to the placenta, surgical planning in the event of a planned cesarean section or obstruction of the birth canal. In the event of pain, ultrasound and/or MRI can be useful to rule out other intra-abdominal causes, to diagnose fibroids and to evaluate for fibroid torsion [31].
Course of Fibroids in Pregnancy
Given that myomas are estrogen and progesterone sensitive, it has been theorized that fibroids would increase in size during pregnancy, due to elevated serum estrogen levels. However, studies have shown that the majority of myomas undergo no significant change in volume [32]. Interpretation of change in size can be difficult, because myomas often change shape during pregnancy, becoming more flattened and elongated as the uterus grows and stretches [32]. As a result, more recent studies have focused on changes in fibroid volume, rather than diameter which may be misleading. Prospective studies utilizing serial ultrasound to assess the change in fibroids during pregnancy have shown that most (60–78 %) do not increase in volume [33, 34]. Contrary to popular thought, some myomas may even decrease in size throughout the course of pregnancy [32–37]. Data revealed 40–64 % show some decrease in volume, with larger myomas (defined as diameter >4 cm) being more likely to decrease, particularly after 30 weeks [37, 38].
Although there has been some inconsistency in results, fibroid growth during pregnancy is much rarer than once thought. When increases do occur, they tend to be during the first trimester [32–34, 36] and are quite small in magnitude, with the maximum observed difference <25 % of initial volume [33, 34]. Overall, changes in size remain difficult to predict as fibroids may remain unchanged, enlarge or decrease in volume during pregnancy. Nonetheless, these changes are typically small with limited clinical implications.
Few studies have assessed change in fibroids extending into the post-partum period, but available data suggests a decrease in size after delivery [32, 33]. In a prospective study by Laughlin et al., 79 % of identified fibroids decreased in size compared to their early-pregnancy scan [35]. There was a greater change in diameter of fibroids in the lower uterine segment, where there is a larger degree of uterine stretching. However, this study calculated change in size from measured diameter, which may overestimate the true change in fibroid volume, as discussed previously [35].
Complications of Fibroids in Pregnancy
Most women with fibroids remain asymptomatic with uncomplicated pregnancies. While the true effects of fibroids on pregnancy remain uncertain, it has been estimated that 10–40 % of patients with myomas will experience a complication [39].
Obstetric Complications
First Trimester Complications
As outlined above, there has been extensive research examining the effects of fibroids on fertility. There have been comparatively few studies, however, evaluating the effects of fibroids on recurrent first trimester pregnancy loss, and what studies there are do not show that the majority of fibroids can be implicated as a cause of this devastating condition. Despite the lack of evidence, there have been a number of proposed mechanisms linking fibroids to recurrent pregnancy loss, all centered on fibroids as a cause of decreased regional blood flow [40].
As in infertility research, the majority of data concerning fibroids related to recurrent pregnancy loss compare women before and after myomectomy [41, 42], or from a compilation of studies examining women undergoing IVF cycles for infertility. One retrospective study concluded that fibroids were associated with an increased rate of pregnancy loss by determining a 60 % rate of miscarriage in women with fibroids that decreased to 24 % after myomectomy [42]. Other studies have consistently shown that submucosal fibroids have the greatest effect on first trimester pregnancy loss when compared to fibroids in other locations, and subsequently should be removed to improve pregnancy outcomes [13, 15, 16, 43]. Generally, submucosal fibroids are singular and smaller than their intramural counterparts and generally are amenable to hysteroscopic resection, making the recommendation for removal an easier decision since the risk profile is low [23, 44–47].
Because the data is inconclusive on whether intramural fibroids may contribute to recurrent spontaneous first trimester pregnancy loss, the risk-benefit ratio of intra-abdominal surgery must be considered when evaluating these patients for treatment. When fibroids have a definitive effect on the intrauterine cavity, they may warrant removal to assist in reproductive outcomes, and when they clearly are separate, removal may not provide any therapeutic benefit [48].
Second and Third Trimester Complications
The most common second and third trimester obstetric complications reported are pain, preterm labor, and fetal malpresentation. Studies have also questioned a relationship between fibroids and placental abruption, placenta previa and intrauterine growth restriction, with conflicting results. Factors associated with a higher risk of complications are fibroid size, location, number, and location relative to placenta implantation.
Fetal malpresentation is one of the more commonly cited outcomes associated with fibroids in pregnancy [5, 49]. Fibroids, particularly those that are submucosal, can distort the uterine cavity and lead to fetal malpresentation [3]. Cumulative data from a review showed 13 % of patients with fibroids had malpresentation, compared to 4.5 % in non-fibroid pregnancies (OR, 2.9; 95 % CI, 2.6–3.2) [10]. This risk, specifically breech presentation, was further increased with larger fibroid volume (>100 mm3) [5] or size (>10 cm) [49].
Uterine fibroids have been associated with an increased risk of preterm labor and delivery (<37 weeks) [49–51]. Studies have indicated a positive correlation with preterm delivery and myoma volume [52]. It has also been suggested that the presence of multiple fibroids may be associated with a higher risk of preterm contractions [50]. The mechanism behind this association is not well understood. One popular theory suggests that the presence of myomas inhibit normal distension of the uterus [5, 49].
Fibroids have been investigated as a cause of intrauterine growth restriction. Most studies have shown no correlation [5, 50]. However, evidence is inconsistent and some authors report a higher incidence of small-for-gestational age infants with increased fibroid volume [10].
Similarly, there are conflicting results regarding placental abnormalities in the presence of fibroids. Some studies report greater risk of placental abruption with fibroids. A more significant risk is suggested with those fibroids that have an increased volume or retroplacental location [3, 10]. Other studies challenge these results, with no observed correlation [52]. There is no evidence to support an association with placenta previa, despite belief that disruption of the uterine cavity could result in lower uterine placental implantation [10].
Non-Obstetric Complications
The majority of fibroids are asymptomatic during pregnancy and do not require treatment, however, when fibroids undergo ‘red-degeneration,’ torsion or impaction, they may be the cause of severe localized abdominal pain and require immediate workup and treatment in pregnancy.
Red or carneous degeneration is the most common non-obstetric complication of fibroids in pregnancy and can occur in up to 5 % of pregnant women with fibroids [52]. Corresponding with the times of greatest growth, red degeneration most commonly occurs in the first or early second trimester of pregnancy. There are a few proposed pathophysiologic mechanisms as to why red degeneration causes severe pain. One is that with rapid growth seen in early pregnancy, the fibroid outgrows its blood supply leading to ischemia, necrosis and infarction [53, 54]. Another proposed mechanism is that the shift in architecture of the growing uterus can cause kinking, distortion or obstruction of the blood supply to the fibroid, even in the absence of significant growth [1]. Along with the two above mechanisms is the thought that the release of prostaglandins from cellular damage associated with fibroid infarction may result in severe pain.
The diagnosis of red degeneration is often made clinically by acute onset localized abdominal pain over a known fibroid location. Pain is the defining feature, but it may also be associated with nausea, vomiting, low-grade fever, and leukocytosis. The diagnosis is one of exclusion, and complications of pregnancy such as preterm labor, placental abruption, pyelonephritis and other intra-abdominal pathology such as appendicitis, nephrolithiasis and constipation must be ruled out. Ultrasound can provide helpful diagnostic clues including anechoic cystic spaces or coarse heterogeneous echogenic patterns within the degenerating fibroids [38].
The mainstay of treatment for red degeneration in pregnancy is conservative management with hospitalization, rest, hydration, and symptomatic analgesia. Non-steroidal anti-inflammatory (NSAID) medications have the best success rates in controlling pain associated with this condition, but must be used with caution, especially in the later part of pregnancy due to neonatal concerns of fetal premature closure of the ductus arteriosis, nephropathy, pulmonary hypertension, necrotizing enterocolitis and oligohydramnios [55]. If a short course (<48 h) of NSAIDs is unsuccessful in managing pain, narcotic pain medication may need to be used sparingly, however has constipating side effects, which may compound the abdominal pain. Epidural analgesia has also been reported with good pain results in severe cases refractory to medical management [56].
Surgical Management of Fibroids in Pregnancy
Although many review articles have been written about the surgical management of fibroids [57], they rarely contain information on the surgical management of fibroids in pregnancy. Conservative management of red degeneration of fibroids in pregnancy is the preferred method of treatment and is often successful. Rarely, though, pain is refractory to medical management or cellular necrosis leads to an acute abdomen with peritoneal signs and surgical management can be considered for treatment of a necrotic or torsed pedunculated fibroid (Fig. 15.8, Table 15.1). Ultrasonographic doppler flow is often inconclusive in the diagnosis of these patients and MRI may be helpful to characterize the size and location of the fibroid as well as the extent of the fibroid stalk for presurgical planning [31]. Careful consideration must be performed prior to taking a patient to surgery as there are increased risks associated with myomectomy during pregnancy. Increased blood flow to and throughout the uterus during pregnancy can lead to significant hemorrhage and surgical entry into the abdomen and uterus can pose fetal risks including preterm labor and fetal distress [58] (Fig. 15.9).
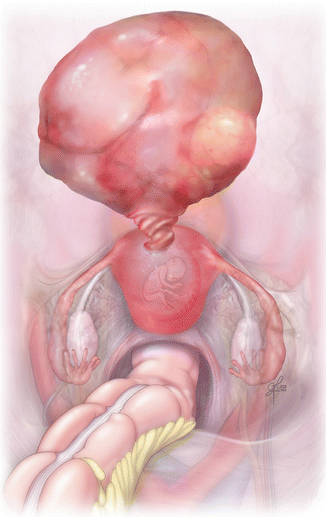
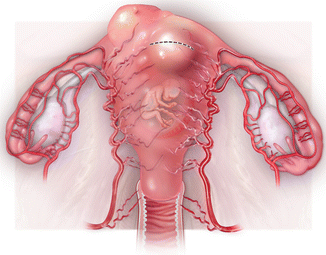

Fig. 15.8
Torsed pedunculated fibroid on a pregnant uterus
Table 15.1
Indications for surgery
Conservative management | Surgical candidate |
|---|---|
Known fibroids | Severe pain with unknown diagnosis |
Non-acute abdomen | Acute abdomen |
Pain responsive to analgesia | Evidence of fibroid torsion on imaging |
Improvement in pain over time | Pain unresponsive to conservative measures |

Fig. 15.9
Blood supply to a pregnant uterus
Previously, laparotomy had been the surgical method of choice for myomectomy during pregnancy due to the concern of entry and insufflation of a gravid abdomen [58–61]. However, more recently there have been multiple case reports of successful minimally invasive laparoscopic myomectomies during the first and second trimesters of pregnancy [62–66] allowing for shorter recovery times, less blood loss, earlier ambulation and less narcotic use post-operatively. There are certain precautions and special considerations proposed for minimally invasive surgery in this unique surgical population (Table 15.2).
Table 15.2
Special considerations for laparoscopic surgery in pregnant patients
Consideration | Options |
|---|---|
Patient positioning | Left lateral tilt |
Initial port placement | Umbilical |
Supra-umbilical | |
Left upper quadrant | |
Mode of entry | Hassan technique |
Verees needle | |
Optiveiw trocar | |
Number of additional ports | 2–4 |
Operative technique | Decreased insufflation pressure |
Decreased Trendelenburg | |
Slow position changes | |
Decreased uterine manipulation | |
Method of fibroid ischemia | Coagulation |
Suture/endoloop | |
Vascular stapler | |
Fibroid removal | Mini-laparotomy |
Morcellation | |
Fetal monitoring | None, intermittent or continuous based on gestational age |
The choice of trocar placement and entry must be carefully considered due to the underlying gravid uterus. Umbilical, supraumbilical or left upper quadrant point of entry have been described based on fundal height and fibroid location. Case reports describing laparoscopic myomectomy at 11, 14 and 25 weeks reported infraumbilical, umbilical and 5 cm supraumbilical port entry respectively [62, 63, 66]. Method of entry including initial Verees needle for pneumoperitoneum followed by blind entry and use of the ‘Hassan’ or open technique of laparoscopic entry have both been described based on surgeon preference [62–66]. While there has not been extensive experience with laparoscopic myomectomy during pregnancy, laparoscopic cholecystectomy and appendectomy have been performed for many years with good success rates and few complications and both methods of entry appear safe [67]. Despite different preferences in initial entry, it is agreed upon that additional ports must be placed under direct visualization. The choice of number and placement of additional ports is again dependent on surgeon preference, with the majority of cases describing two to four additional ports based on fibroid size and location for retraction, visualization and ease of suturing. Additional ports may be helpful to minimize manipulation of the uterus with utmost care during retraction, pushing and pulling as to not disrupt blood flow or damage the uterus (Fig. 15.10).
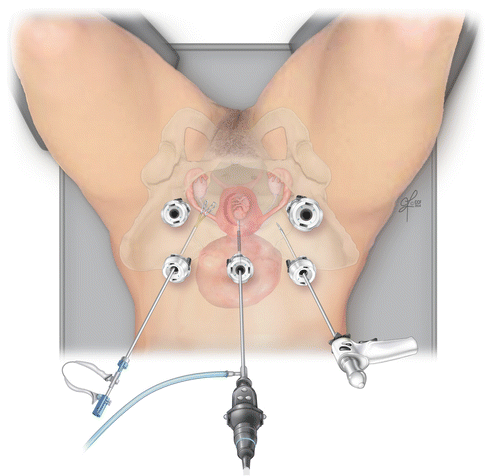

Fig. 15.10
Careful consideration of port placement in a pregnant patient
The choice of a 0 or 30° laparoscope may also be used to assist in visualization and decrease manipulation of the uterus.
Patient positioning before and during surgery can help to decrease the risk of utero-placental insufficiency. Initial positioning of the patient in left-lateral tilt and slow change in and out of the least possible amount of Trendelenburg needed to complete the surgery may assist in keeping adequate blood flow to the uterus. In addition, slow insufflation of carbon dioxide gas at lower pressures (10 mmHg vs. 15 mmHg) can help to avoid carbon dioxide absorption leading to acid-base disturbances [66].
There are a number of intra-op surgical considerations as well. The location of laparoscopic myomectomy incision should be well planned with consideration of imminent suture closure, as it is generally considered easier to suture in the horizontal plane. Multiple methods of fibroid ischemia and separation from the uterus have been described including careful dissection with cautery at the base [63], strangulation with Monocryl suture [62], use of an Endoloop suture [67] and the use of an endoscopic vascular stapling device [66]. All methods attempt to minimize blood loss while limiting the use of monopolar electric and bipolar current to decrease the risk of electro-surgical damage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


