Fig. 5.1
Treatment options for uterine fibroids
A recent study showed that the median serum AMH levels and median AFC per ovary were significantly lowered after Uterine Arterial Embolization (UAE) compared to women who had undergone LM (Laparoscopic Myomectomy) concluding that this could have an adverse impact on future response to fertility treatment and/or fecundity [40]. As the safety and effectiveness of UAE has not been established for women with myomas seeking to maintain or improve their fertility, it should not be recommended till further evidence is available.
Although studies claim that treatment of symptomatic uterine myomas with MRI guided Ultrasound (MgRfoUS) improves both QOL and subsequent fertility, further evidence should be explored before its application in women of reproductive age [41].
Medical treatment for myomas does not improve infertility. Preoperative medical treatment with a GnRH agonist should be considered for women who are anemic and those who might be candidates for a less invasive procedure if the volume of their myoma(s) was moderately smaller (The Practice Committee of the American Society for Reproductive Medicine in collaboration with The Society of Reproductive Surgeons).
However, according to the specific needs and with future evidence based medicine UAE, focused Ultrasound applied under MRI and the medical treatment with the progesterone modulator Ulipristal Acetate (5 mg) might be considered [42]. It has recently gained importance in the treatment of menstrual disturbances due to submucosal and intramural fibroids.
Myomectomy and Fertility: Laparoscopic and Hysteroscopic Resection of Fibroids
General Aspects
Based on immunohistochemical findings, it is proposed to remove fibroids in women seeking pregnancy whilst respecting the pseudocapsule by neurofibre sparing in the incision site. We published that this is of utmost importance for optimal muscular healing and myometrial function in future pregnancies. In fibroids detected under a size of 5–6 cm in diameter especially in young women wanting to achieve pregnancies, the myomectomy should be performed before the myoma reaches a size causing compression of the surrounding tissues and uterine distortion, which may result in the loss of regenerative potential [43].
It is good surgical and clinical practice to perform a hysteroscopy before advancing to the laparoscopic myomectomy. The advantage of the hysteroscopy is that, as this is a patient seeking fertility, endometrial pathology can be identified and corrected and intra-cavity extension of the fibroid can be noted. A chrompertubation should be performed before proceeding to the myomectomy. It is advisable to use a uterine manipulator to stabilize the mobile uterus during a myomectomy.
A longitudinal incision is usually preferred on the uterus for a myomectomy but, if the myoma extends laterally, a horizontal incision is also acceptable. If the uterine cavity is entered during the procedure, it just has to be sutured additionally in a special layer. If the fibroid is posterior, there might be an increased risk of adhesion formation and an anti-adhesive strategy needs to be adopted. The patient should always be informed that there might be a rare possibility to convert the surgery to a laparotomy.
The Practice Committee of the American Society for Reproductive Medicine in collaboration with The Society of Reproductive Surgeons concluded that Myomectomy is a relatively safe surgical procedure associated with few serious complications. However, postoperative adhesions are common after abdominal myomectomy and pose a significant potential threat to subsequent fertility. Hence, a laparoscopic and/or hysteroscopic myomectomy should be preferred to an abdominal. However, each surgeon should determine his or her own criteria for laparoscopic myomectomy.
Post-surgery, the patient is usually advised to attempt pregnancy after 3 months to give adequate time for the uterine scar to heal.
Laparoscopic Stepwise Enucleation of an Intramural Fibroid and Uterine Reconstruction
Figures 5.2, 5.3, 5.4, 5.5, 5.6, 5.7, and 5.8 give a detailed diagnostic and surgical description of myomectomy and surgical reconstruction of the uterine wall.
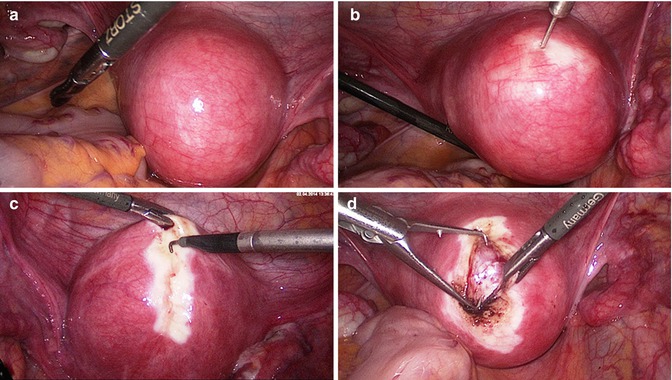
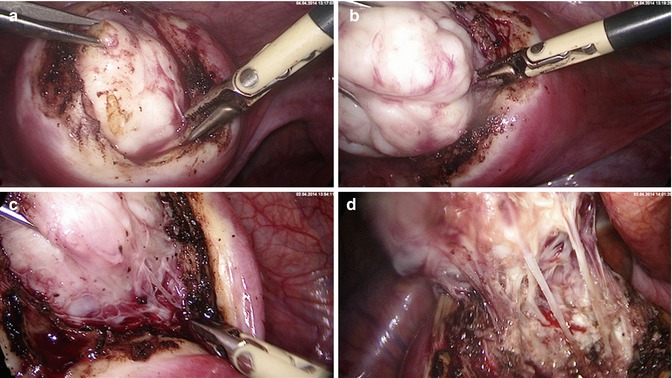
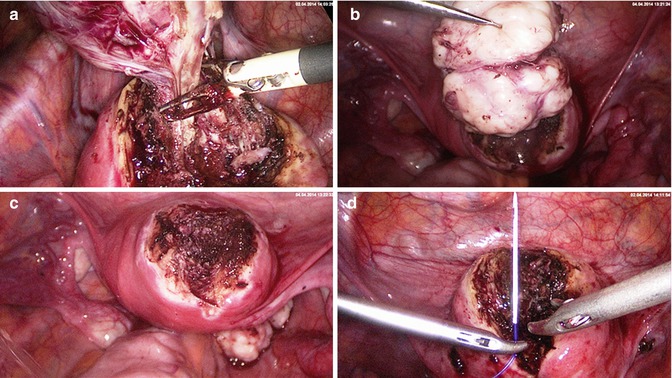
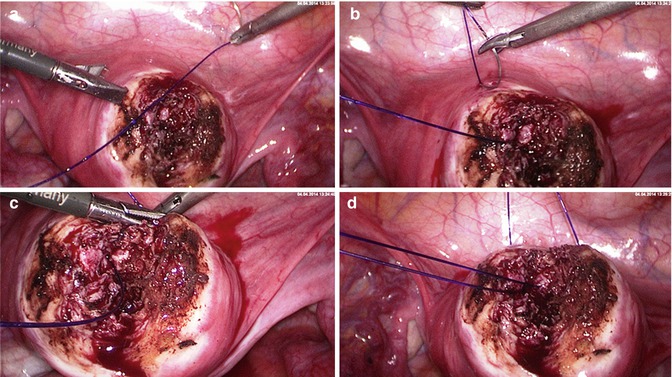
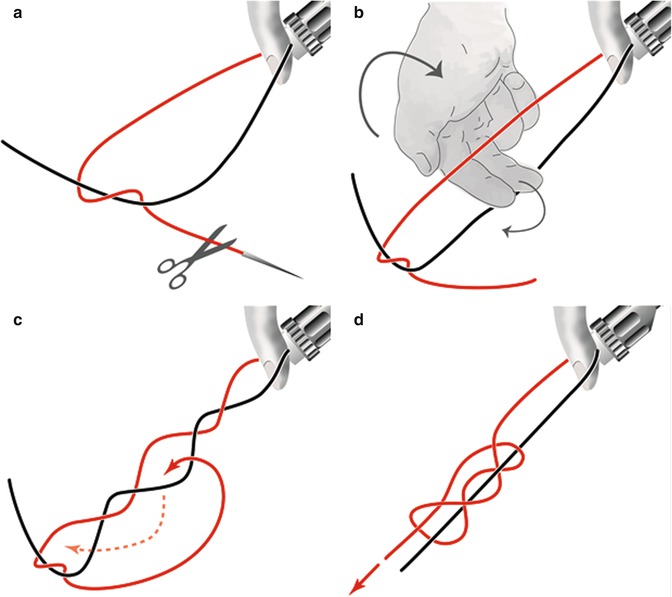
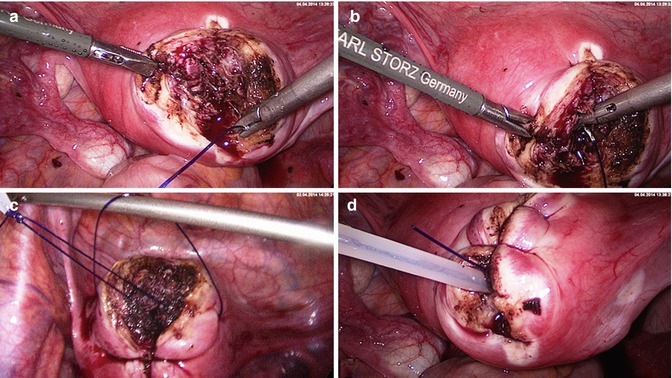
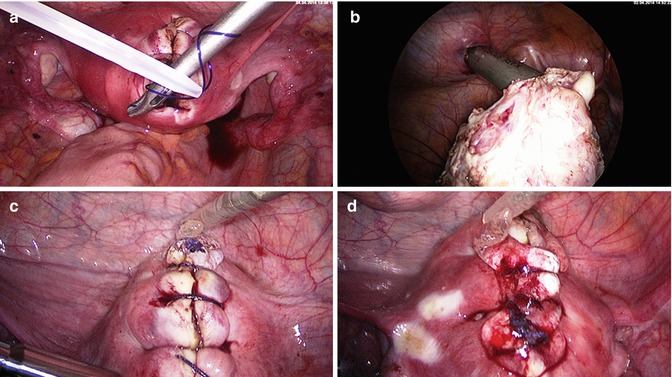

Fig. 5.2
Laparoscopic myoma enucleation. (a) Situs of a fundal/anterior wall fibroid. (b) Prophylactic hemostasis with 1:100 diluted vasopressin solution (Gylcilpressin) in separate wells. The injection intends to separate the pseudocapsule from the fibroid and reduces bleedings. (c) Bipolar superficial coagulation of the longitudinal incision strip and opening of the uterine wall with the monopolar hook or needle till the fibroid surface. (d) Grasping of the fibroid and beginning of the enucleation. The pseudocapsule remains within the uterine wall and is pushed off bluntly

Fig. 5.3
Laparoscopic myoma enucleation. (a) Traction of the fibroid with a tenaculum and blunt delineation from the capsule. (b) Focal bipolar coagulation of basic vessels. (c) Continuous enucleation of the fibroid under traction and specific coagulation of capsule fibers containing vessels. (d) Magnification of remaining capsule fibers to be coagulated and cut

Fig. 5.4
Laparoscopic myoma enucleation. (a) Final coagulation of the capsule vessels. (b) Double belly fibroid after complete enucleation. (c) Minimal coagulation of bleeding vessels under suction and irrigation. (d) Approximation of wound edges with either straight or round sharp needle and a monofilar late resorbable suture

Fig. 5.5
Laparoscopic myoma enucleation. (a) Advantage of round needle stich. The wound angle is elevated safely and completely by elevating it with a Manhes-forceps. Deeper layers of the myometrium can be grasped more easily using a round needle. (b) Needle exit and simplified regrasping with the right needle holder. (c) Final stich to invert the knot. (d) Extirpation of the needle and completing the extracorporal knot and preparing to push down the extracorporal knot

Fig. 5.6
Performance of the extracorporal “von Leffern” knot. (a) Pulling out the suture, removing the needle, half hitch. (b) Holding the knot with the left hand and reaching over with the right hand. (c) Grasping the short end from below and leading it back, exiting before the half hitch. (d) Turning back the knot. Holding the straight suture and tightening the knot

Fig. 5.7
Laparoscopic myoma enucleation. (a) Second single stich starting as deep as possible in the uterine wound. (b) Exiting of the needle on the left wound margin (just next to the Manhes forceps). (c) Completing of the stich and preparation of the extracorporal von Leffern knot. The needle holder elevates the thread to avoid tearing of the uterine wall while pulling through the monofilar thread (PDS). (d) Pushing down the extracorporal performed knot with a plastic push-rod in the depth of the wound to dump the knot minimizing the external suture part

Fig. 5.8
Laparoscopic myoma enucleation. (a) Intracorporal safety knot of the performed extracorporal knot. (b) Morcellation of the fibroid with the Rotocut morcellator (Storz) in an apple peeling manner. (c) Final situs showing the extracorporal sutures to adapt the uterine wound edges. (d) Application of Hyalobarrier® (Nordic Pharma) for adhesion prevention
This step wise description of laparoscopic myoma enucleation as an intracapsular approach with an adequate reconstruction of the uterine wall gives the patients a good start for further fertility results. The adaption of wound edges may be in one, two or three layers depending of the situation. If the uterine cavity has been opened, an extra layer of sutures has to be applied. Conventional or barbed sutures are acceptable.
Hysteroscopic Myoma Enucleation Has to Be Performed According to the Depth of Infiltration into the Myometrium
Submucous myomas may cause serious implantation problems and raise the frequency of abortions. The best time of surgery is the early phase in the cycle without bleeding. Saline Infusion Sonography best reveals the fibroid enucleation level and helps to plan the correct surgery which sometimes needs to be combined with a laparoscopic approach [8].
Differentiation of Fibroids and Focal Endometriosis to Adenomatoid Tumors
Focal adenomyosis may create a lot of pain and has to be resected if the patient is below the childbearing age or wants to conceive sooner or later, although hysterectomy best solves the dysmenorrhea of these patients. But, this is of course not an option in infertile women. Focal adenomyosis are of mesothelial origin and affect the epididymis, testis, tunica albuginea, ejaculatory duct, prostate and spermatic cord in men and uterus, ovary and fallopian tubes in women. Cases have been reported of adenomatoid tumors located in the heart, pleura, liver and adrenals [44–47]. Multifocal or multicentric appearance is exceptional [48, 49].
The excision of these lesions is much more difficult than any myomectomy as there is no myoma capsule. Adenomatoid tumors also resemble fibroids without a capsule, sometimes after GnRH analogue treatments. They can also be mistaken for lymphangiomas, metastatic adenocarcinoma and metastasis of other origin.
These tumors form circumscribed tubercular solid masses. A recognizable separating layer or capsule enclosing the lesion is missing, as the tumors are densely adherent to the surrounding tissue. These circumstances make intraoperative preparation difficult and inhibit the definite macroscopic exclusion of a malignant event. Sixty percent of the uterine adenomatoid tumors are subserosal or at least in the external region of the myometrium. As described in our cases, most frequently they are situated in the fundus or in the posterior wall of the uterus [44–46, 50, 51]. The following 2 case reports reflect the complexity of the problem.
Case 1
A 26-year-old Caucasian woman, nulliparous, was transferred for surgery with a recurring symptomatic ovarian cyst. During the gynecological examination, in addition to an unsuspicious-looking cyst on the left ovary, a 2.1 cm diameter well-circumscribed uterine mass located in the posterior wall of the fundus, 1.2 cm from the serosal surface was recorded as a fibroid (Fig. 5.9). This known tumor had been seen by an ultrasound scan 1-year earlier measuring 1 cm in diameter. The patient had a medical history of three laparoscopic surgeries for the enucleation of relapsing functional ovarian cysts with no evidence of endometriosis.
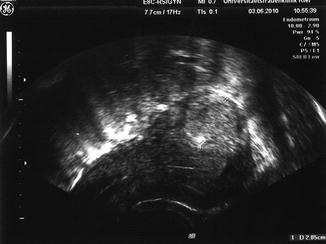

Fig. 5.9
Preoperative transvaginal ultrasound scan showing the typical misleading sonographic picture of a fibroid (case 1)
The patient required a fourth laparoscopy to treat the symptomatic ovarian cyst. Because of the growth of the tumor on the posterior wall, the age of the patient and possible problems in future family planning, it was decided to simultaneously excise the suspected myoma. At laparoscopy, the ovarian cyst was enucleated and the presumed fibroid was resected. Excision of the tumor was difficult as it was smoother than a typical myoma and more difficult to grasp with forceps. The tumor was enucleated with a special instrument which we also use to remove myomas. The usual enucleation performed in fibroid surgery was not possible as there was no capsule separating nodule from the myometrium and the tumor seemed to grow into the orthotope myometric tissue (Fig. 5.10a). After removal of the nodule and the surrounding myometrial layer, the uterine wall was reconstructed in a single layer with reversed and inverted single stitches (Fig. 5.10b). The tumor was removed after intraabdominal morcellation (Fig. 5.10c).
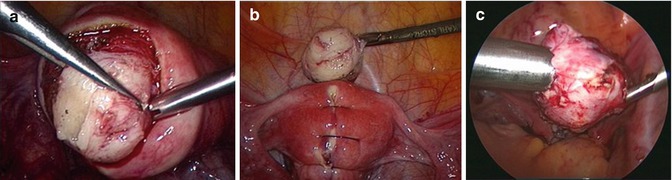

Fig. 5.10
(a) Intraoperative sight of the adenomatoid tumor connected to the surrounding myometrium (case 1). (b) Reconstruction of the uterine wall after excision of the tumor (case 1). (c) Removing the adenomatoid tumor by morcellation (case 2)
Case 2
A 19-year-old nulligravida presented with pain in the lower abdomen for the last 6 months, dyspareunia and pain in the back. Transvaginal ultrasound revealed a well-circumscribed 5 cm mass in the fundus of the uterus. No additional lesions were noted in the pelvis. The patient underwent an uneventful laparoscopic procedure with excision of the tumour and reconstruction of uterus wall.
The post-operative recovery was in each case unremarkable and the patients were discharged 2 days after surgery free of pain. The follow-up period was without pathological findings.
Histological tissue was available from both original tumor specimens. Routine histological studies were performed according to the usual procedures: 4-μm thick sections of formaldehyde-fixed, paraffin-embedded tissue were stained with hematoxylin and eosin (H&E) for the light microscopic histological examination. Immunohistochemistry was performed using the following antibodies: calretinin for staining cells of mesothelial origin and CD34 for marking endothelial cells, KI-67 was used as a proliferation marker [47, 52].
Macroscopically, both tumors showed a white-grey, nodular, non-capsulated surface. The histological examination showed smooth muscle cells of normal myometrium and in between the myometrium tumor elements consisted of slit-like, tubular, cystic or cribriform anastomosing gland-like spaces reminiscent of vascular structures (Fig. 5.11a, b). The lining cells were columnar, cuboidal to flat with bland cytologic features and mitotic activity. The angiomatoid spaces were lined by a single layer of flattened cells with oval or round nuclei, divided by fine connective tissue septa rich in blood vessels with a slight lymphocytic infiltration. The spaces contained cells with slightly eosinophilic cytoplasm and prominent cytoplasmic vacuoles that mimic signet ring cells. The pseudoglandular spaces were surrounded by hyperplastic smooth muscle with a sprinkling of stromal lymphocytes. Nuclei were usually small with inconspicuous nucleoli. Neither atypical nuclei, mitoses or necrosis were found.
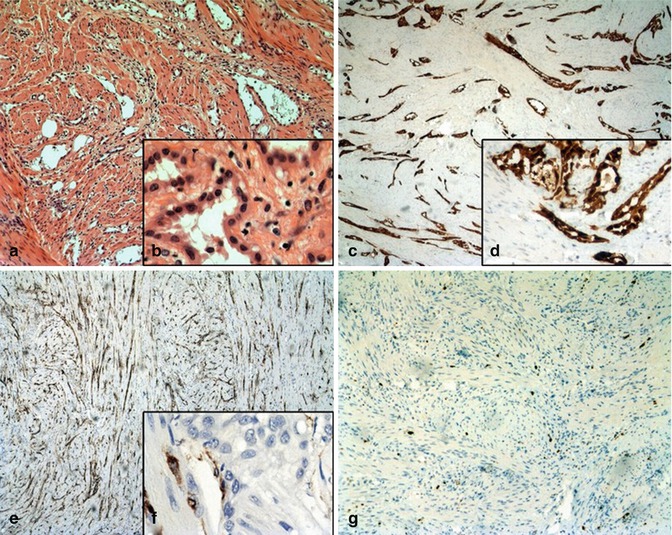

Fig. 5.11
(H&E): (a) low magnification (×25) of the uterine adenomatoid tumor showing the typical tubular-glandular growth pattern and surrounding small muscle cells. (b) The lining cells in typical cuboidal growth pattern with unsuspicious nuclei at high magnification (×400). (Calretintin): (c) showing the strongly positive immunohistochemical calretinin staining of the tumor tissue (×25×). (d) Positive stained tumor cells at high magnification (×400) (CD34): Immunohistochemical CD34 staining (e, f) showing no positive staining of the tumor cells but the surrounding lymphovascular endothelial cells in low and high magnification (×25 and ×400). (Ki–67): Immunohistochemical Ki-67 staining (10×) showing no enhanced mitotic activity of the tumor tissue (g)
Immunohistochemical techniques showed a strong staining of tumor cells with calretinin but no staining with CD34 (Fig. 5.11c, d). However, CD34 marked the endothelial cells and the surrounding orthotopic lymphovascular vessels (Fig. 5.11e, f). The Ki-67 index of the tumor cells was <1 % (Fig. 5.11g).
Adenomatoid tumors occur most commonly during the reproductive years. Nevertheless, they are rare, benign neoplasms, occurring in about 1 % of pathologically examined hysterectomies and are mostly incidental findings [45, 53]. In the majority of the known case reports the diagnosis is made only after pathological examination of other suspected tumor origins. As in our case, most of the adenomatoid tumors remain asymptomatic. Adenomatoid tumors of the uterus can be either subserosal or intramural and can involve the subendometrial myometrium. Due to their anatomical localization, the most common symptoms are pain and menorrhagia or symptoms associated with adenomyosis uteri. Many cases of incidental diagnosis do not show any clinical symptoms at all [45, 46, 52, 54–56]. In females, adenomatoid tumors are mostly situated in the fallopian tubes and the uterus, and less frequently in the ovaries or the periovarial tissue. In males, they occur in the epididymidis, tunica albuginea and testicular parenchyma. Very seldom, adenomatoid tumors are seen in extragenital regions e.g. adrenal gland, omentum majus or liver. Accordingly, the clinical symptoms are similar to those caused by other benign space-consuming lesions in these regions. There is no evidence of recurrence, malignant transformation or metastasis [46].
In the majority of cases preoperative detailed differential diagnostics are the exception as adenomatoid tumors are found incidentally. Despite the well-established microscopic features that distinguish adenomatoid tumors from all other entities, preliminary clinical examination, ultrasound or MRI cannot differentiate adenomatoid tumors from their differential diagnosis [52, 56, 57].
The dissimilarity to uterine fibroids, the most frequent misdiagnosis of uterine adenomatoid tumors, is seen in the intraoperative complexity of the separation between tumor and myometrium. Mitsumori et al. report two cases of adenomatoid tumors of the uterus that also imitated leiomyoma and were only diagnosed postoperatively [57]. Histologically, fibroids have their own capsule of connective tissue. This is missing in adenomatoid tumors [49] and it is, therefore, necessary to include a layer of unaffected myometrium when operating adenomatoid tumors. Nevertheless, laparoscopic surgery for the treatment of adenomatoid tumors is feasible and recommended. In contrast to adenomyosis uteri, there is no histological infiltration of endometrial glands and stroma into the myometrial tissue. Adenomyosis uteri in its primary and disseminated forms, infiltrates the entire myometrial wall. A more disseminated spreading of adenomatoid tumor has been reported in women with immunosuppression [58].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


