Uterine Cancer
David G. Mutch
The uterus and endometrium are unique organs. Almost any type of tissue histology can and does arise from these pluripotent tissues. This chapter will present premalignant and malignant conditions of the uterus. This includes the premalignant condition of endometrial hyperplasia as well as the frankly malignant conditions of the uterus that comprise the following two broad categories: (a) epithelial cancer and (b) mesenchymal tumors. Both of these types of malignancies develop within the lining or the body of the uterus, and this chapter will describe the epidemiology, staging, and treatment of these cancers. The epithelial cancers are primarily comprised of endometrioid, clear cell, and papillary serous tumors. The mesenchymal tumors are less common and are comprised of three broad categories: (a) mixed Müllerian tumors, (b) stromal tumors, and (c) leiomyosarcomas.
Endometrial Hyperplasia
Classification
Endometrial hyperplasia is an abnormal condition that usually represents an overgrowth of the endometrium. This terminology encompasses a wide variety of conditions. Some of these are clearly benign and some potentially malignant. Unfortunately, there have been so many different classifications over the years that there is significant confusion about the meaning of the term endometrial hyperplasia. For many years, it has been suggested that endometrial hyperplasia reflects the histologic representation of the continuum between normal proliferating endometrium and adenocarcinoma in situ. This theory was based on studies first reported by Gusberg and Kaplan in 1963. In that study, the authors reported that 20% of patients who had a hysterectomy were found to have a coexisting adenocarcinoma and that endometrial cancer developed in almost 12% of the remaining patients, with an average follow-up of 5.3 years. They concluded that the risk of cancer was significantly higher in women with endometrial hyperplasia than those without and that after 10 years the cumulative risk for cancer was approximately 30%.
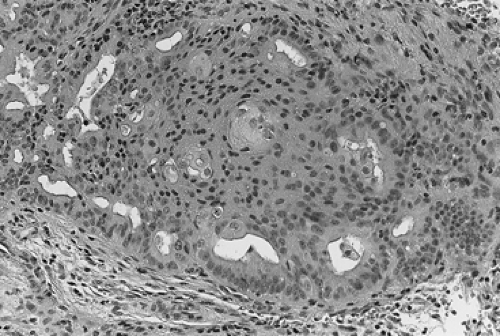
During the ensuing years, several different classification schemes were developed, which resulted in increased confusion regarding both diagnostic criteria and prognosis of the various subtypes of hyperplasia. In the early 1990s, the International Society of Gynecological Pathologists endorsed a classification that used both architectural features (the degree of glandular crowding and complexity) and cytologic features, especially cellular atypia. In this classification system, simple hyperplasia is defined as abnormally thickened endometrium with histologic evidence of an increased ratio of glands to stroma; the glands are cystically dilated and somewhat irregular with some infolding and budding. Complex hyperplasia represents glandular crowding with even less intervening stroma, and the glands show significant infolding and budding (Fig. 60.1). Atypical hyperplasia refers to either simple or complex architectural patterns, in which the cells lining the glands show loss of polarity, nuclear enlargement with increased nucleus-to-cytoplasm ratio and prominent nucleoli, and irregularly condensed chromatin (Fig. 60.2). Cystic hyperplasia is a benign condition arising from inactive endometrium and is not premalignant. It is the atypical changes that portend a worse prognosis in terms of development of malignancy in these premalignant conditions.
Since the study by Gusberg and Kaplan, additional studies have clarified some of the questions regarding this disorder. Using the new criteria, Kurman and colleagues reported that the risk of progression of endometrial hyperplasia to cancer varied according to the subtype. In this study of 170 patients, untreated endometrial hyperplasia regressed spontaneously in 74% and remained stable for more than 10 years in 18%. The risk of progression to cancer was 1% for patients with simple hyperplasia without atypia, 3% for those with complex hyperplasia without atypia,
8% for those with atypical simple hyperplasia, and 29% for patients with complex atypical adenomatous hyperplasia (Table 60.1). Finally, in 2005, data from the Gynecologic Oncology Group (GOG) was reported. GOG 167 was a protocol designed to follow the natural history of this disease and to assess malignant potential in a prospectively acquired cohort of patients with complex atypical hyperplasia. Several interesting findings were published from this data: (a) the risk of concomitant cancer was approximately 40%, (b) there was little difference in the accuracy of the pathology reading between community pathologist review and that of an academic pathologist, and (c) more extensive sampling by using dilation and curettage (D&C) in addition to an endometrial biopsy did not change the accuracy of the diagnosis. Therefore, once childbearing is complete, definitive surgery is probably the best option for treatment of women with complex atypical hyperplasia, and a simple endometrial biopsy appears to adequately make the diagnosis.
8% for those with atypical simple hyperplasia, and 29% for patients with complex atypical adenomatous hyperplasia (Table 60.1). Finally, in 2005, data from the Gynecologic Oncology Group (GOG) was reported. GOG 167 was a protocol designed to follow the natural history of this disease and to assess malignant potential in a prospectively acquired cohort of patients with complex atypical hyperplasia. Several interesting findings were published from this data: (a) the risk of concomitant cancer was approximately 40%, (b) there was little difference in the accuracy of the pathology reading between community pathologist review and that of an academic pathologist, and (c) more extensive sampling by using dilation and curettage (D&C) in addition to an endometrial biopsy did not change the accuracy of the diagnosis. Therefore, once childbearing is complete, definitive surgery is probably the best option for treatment of women with complex atypical hyperplasia, and a simple endometrial biopsy appears to adequately make the diagnosis.
Clinical Picture and Diagnosis
Endometrial hyperplasia often becomes apparent with irregular vaginal bleeding. For this reason, this symptom should be evaluated with liberal use of the endometrial biopsy. Endometrial hyperplasia is commonly associated with a history of unopposed estrogen exposure, either exogenous or endogenous. In premenopausal women, it is associated with obesity and anovulation. Therefore, women with known polycystic ovary syndrome or known anovulation are at increased risk for developing this disease, as are obese women. Any abnormal bleeding should be investigated in the postmenopausal woman, but conditions that would increase the suspicion of an abnormal endometrium in the premenopausal woman are unopposed estrogen exposure from obesity or oral intake. Irregular bleeding in this setting should be evaluated with an endometrial biopsy.
TABLE 60.1 Natural History of Premalignant Endometrial Conditions | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 Figure 60.2 Complex hyperplasia with atypia. Closely packed, irregular glands demonstrate cytologic atypia in the form of loss of polarity and rounding up of the nuclei and nucleoli. |
Cervical cytologic screening techniques are not a reliable means to diagnose endometrial abnormalities and should not be used to screen for endometrial abnormalities. Nonetheless, the Pap smear can reveal endometrial abnormalities. Atypical glandular cells of undetermined significance (AGUS) should be evaluated aggressively, because the risk of having underlying endometrial pathology is high. Endometrial biopsy clearly should be included in the evaluation of this abnormal cytology, especially in women over the age of 35. An endometrial biopsy is reported to be 97% as sensitive as a formal D&C. Therefore, a D&C to evaluate these abnormalities is usually not necessary and should be used only when an endometrial biopsy
cannot be performed; when persistent bleeding is unexplained by the biopsy; or when there is some uterine structural abnormality suspected, such as a polyp.
cannot be performed; when persistent bleeding is unexplained by the biopsy; or when there is some uterine structural abnormality suspected, such as a polyp.
Management
Therapy for endometrial hyperplasia must be individualized and depends on histologic criteria, predisposing factors, patient age, and desire to maintain fertility. In addition to differences in overall prognosis, the various subtypes of endometrial hyperplasia also respond differently to progestin therapy. Ferenczy and Gelfand reported on the results of progestin therapy in 85 postmenopausal women with endometrial hyperplasia. Patients who had hyperplasia without atypia enjoyed complete reversal of the abnormality following treatment with medroxyprogesterone acetate, 10 to 20 mg daily. Patients with cellular atypia noted only a 50% response rate to the same treatment regimen. In patients with atypical hyperplasia, there was also an increased risk of recurrent hyperplasia or cancer after completion of the progestin therapy when compared with those who had hyperplasia without atypia (50% vs. 6%). Premenopausal women can also be treated with oral contraceptives for 3 months if they have no significant contraindications to their use.
Most studies suggest that cancer will develop eventually in about 20% to 30% of patients with complex atypical adenomatous hyperplasia, but some have suggested a risk as high as 82% for those with untreated atypical hyperplasia. The risk of progression appears to be higher for postmenopausal than premenopausal women. The mean duration of progression from endometrial hyperplasia to carcinoma is about 10 years for lesions without atypia and 4 years for atypical lesions. In patients with atypical hyperplasia diagnosed by an endometrial biopsy specimen, a formal D&C should be considered to rule out a coexisting adenocarcinoma. It has been suggested that if gland epithelium is found within the stroma (i.e., stromal invasion) of the curettage specimen, even if the diagnosis is hyperplasia, there is a significant chance that the uterus still contains an endometrial cancer. If a diagnosis of carcinoma in situ is made by endometrial biopsy or D&C, in almost all circumstances a hysterectomy should be performed, because this is not a clearly reproducible diagnosis and actually may represent early invasive cancer or sampling error.
Progestational therapy is very effective in reversing endometrial hyperplasia without atypia. For these patients, either cyclic or continuous therapy is appropriate, using medroxyprogesterone acetate, 10 to 20 mg per day, or megestrol acetate (Megace), 20 to 40 mg per day, for either 14 days each month or daily. Therapy should be continued for 3 months and then the endometrium should again be sampled to document response. The hyperplasia will revert to normal in 75% to 90% of patients treated with progestins. In patients who desire pregnancy, ovulation induction can be considered by using either clomiphene or menotropins (Pergonal). If a patient does not desire pregnancy, long-term oral contraceptive therapy should be considered. In those patients with persistent hyperplasia, definitive surgery should strongly be considered.
Patients with atypical hyperplasia often will opt for a hysterectomy and bilateral salpingo-oophorectomy when made aware of the risk of coexisting adenocarcinoma (approximately 40%) and the malignant potential of these lesions. A truly postmenopausal woman (last menses 2 or more years ago) should be encouraged to undergo hysterectomy, with progestin therapy reserved for patients with severe medical problems that would make them very poor surgical candidates or patients who desire preservation of childbearing potential. If the decision is made to treat a patient medically, daily progestin therapy for 3 months is recommended, followed by repeat endometrial sampling. If hyperplasia persists in these patients, a D&C should be performed to rule out a coexisting malignancy or a hysterectomy should be performed. At the time of hysterectomy, the uterus should be opened intraoperatively, with frozen section if indicated to document the presence and extent of any malignancy so that surgical staging can be performed at the same time, if appropriate.
An interesting, but still experimental, approach is insertion of an intrauterine contraceptive progesterone system (ICPS), which releases continuous therapeutic doses of progesterone or levonorgestrel. Until more experience is gained with the ICPS, it should not be employed outside a research setting. Two preliminary studies reported on the use of danazol (Danocrine) to treat women with adenomatous hyperplasia, and in both studies, all women treated were noted to have reversal of the hyperplasia on repeat endometrial sampling. In the premenopausal women, menses resumed within 1 to 2 months following therapy. In the first study, atrophic changes were noted on repeat endometrial sampling even though the patients had normal serum estradiol levels, suggesting a direct effect on the endometrium.
A relatively recently described risk factor for endometrial hyperplasia and carcinoma is the use of tamoxifen citrate (Nolvadex) as an adjuvant treatment for breast cancer. Tamoxifen has been associated with a six- to sevenfold risk of developing endometrial cancer and an increased risk of endometrial hyperplasia, polyps, and growth of fibroids. The best method for monitoring women taking tamoxifen is unknown. Obviously, patients should have an annual pelvic examination and Pap smear. Endometrial sampling should be performed in all patients with abnormal uterine bleeding. Because tamoxifen acts as a weak estrogen, it is not unreasonable to consider annual endometrial biopsy or sonographic evaluation of endometrial stripe thickness. This recommendation, however, is not based on prospective studies but rather on an intuitive approach. According to current information, women taking tamoxifen have >84% chance of developing a thickened endometrium, making this technique relatively nonspecific in evaluating the endometrium for pathology. Based on published studies, sonography is useful only for ruling out
significant pathology if the endometrial stripe is <5 mm in thickness. Therefore, this may not be useful in screening women on tamoxifen.
significant pathology if the endometrial stripe is <5 mm in thickness. Therefore, this may not be useful in screening women on tamoxifen.
In summary, the management of endometrial hyperplasia should be individualized, depending on the histologic findings and the patient’s age health and reproductive desires. Treatment options include hormone therapy and surgery. However, if the patient has completed childbearing and the diagnosis of complex hyperplasia with atypia has been made, the data acquired from GOG 167 would encourage the recommendation of total abdominal hysterectomy and bilateral salpingo-oophorectomy. Furthermore, the increasing use of tamoxifen should alert gynecologists to expect more cases of both benign and malignant changes within the endometrium, and they should be prepared to evaluate them for these abnormalities.
Endometrial Carcinoma
Incidence and Epidemiology
The American Cancer Society estimated that there were approximately 39,079 cases of epithelial endometrial carcinoma in the United States during 2007, accounting for about 7% of all malignancies in women. It ranks seventh in the cause of death from cancer in women and accounts for about 7,000 to 7,500 deaths each year. At this time, endometrial cancer is the fourth most common cancer in women and is the most common gynecologic cancer. Occurring more frequently than endometrial cancer are lung, breast, and colon cancers. Endometrial cancer generally is thought to be a disease of postmenopausal women. However, one fourth of the cases may occur in women who are premenopausal, and about 5% occur in women under the age of 40. Generally, the prognosis is good, with an overall survival rate for all cases of about 75%. Management of this disease has evolved significantly over the past half-century. Preoperative radiation therapy followed by hysterectomy was the standard before the early 1980s, but now almost all patients are staged surgically, and postoperative management is tailored to the risk factors identified at the time of surgical staging. In 1988, this primary operative approach was formalized by the International Federation of Gynecology and Obstetrics (FIGO) and now requires surgical staging to accurately assign stages and determine appropriate treatment and progress of this disease (Table 60.2).
TABLE 60.2 International Federation of Obstetrics and Gynecology Staging for Carcinoma of the Corpus Uteri | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
According to data from the American Cancer Society, the number of deaths from endometrial cancer was about 4,000 in 1990. In 2007, the mortality from endometrial
cancer increased to 7,400. The exact reason for this is increase is unclear. Many suggest that this increased incidence is caused by the increased use of estrogen replacement therapy (ERT). Several known pieces of information can be used to argue against this. First, death from estrogen-induced endometrial cancer is rare. These cancers usually are well differentiated and carry a low mortality rate. Also, the rate of endometrial cancer is increasing in countries such as Norway and Czechoslovakia, yet estrogens rarely are prescribed in these countries. The incidence of endometrial carcinoma has increased over the last 50 years, most likely because of the aging population, the increased frequency of certain predisposing conditions such as obesity, and improved methods of diagnosis.
cancer increased to 7,400. The exact reason for this is increase is unclear. Many suggest that this increased incidence is caused by the increased use of estrogen replacement therapy (ERT). Several known pieces of information can be used to argue against this. First, death from estrogen-induced endometrial cancer is rare. These cancers usually are well differentiated and carry a low mortality rate. Also, the rate of endometrial cancer is increasing in countries such as Norway and Czechoslovakia, yet estrogens rarely are prescribed in these countries. The incidence of endometrial carcinoma has increased over the last 50 years, most likely because of the aging population, the increased frequency of certain predisposing conditions such as obesity, and improved methods of diagnosis.
Adenocarcinoma of the endometrium is mainly a malignancy of postmenopausal women and is increasingly virulent with advancing age. Peak age at diagnosis is between 50 and 65 years, and approximately 25% of all cases of endometrial carcinoma are diagnosed in premenopausal women and 5% in women younger than 40 years. Usually, but not always, these young women are either obese, chronically anovulatory, or both. Women with endometrial cancer diagnosed at an early age should be queried about family history because endometrial cancer is the most commonly inherited gynecologic malignancy. It is the second most common cancer described in hereditary nonpolyposis colon cancer (HNPCC), or Lynch syndrome.
Bohkman proposed that there are two types of endometrial cancers, designated type I and type II. Type I endometrial cancer is estrogen dependent and is thought to progress typically from hyperplasia to frank cancer in a stepwise fashion. This type of malignancy typically occurs in younger, perimenopausal women with a history of exposure to unopposed estrogen, either exogenous or endogenous. These tumors tend to arise in regions of hyperplasia, tend to be well differentiated, and tend to be associated with a more favorable prognosis. The latter type of cancer (type II) tends to occur in older women without estrogen stimulation of the endometrium, is not often associated with endometrial hyperplasia, and is more commonly associated with poorly differentiated cancer or those of unusual histologic types. It generally carries a worse prognosis. Because cancer is a genetic disease and it is now realized that it develops as the result of an accumulation of mutations in genes necessary for normal cellular function, the pathways necessary for the development of these two types of cancers may be different and is an area of intense investigation by many researchers.
The main risk factor for endometrial adenocarcinoma is long-term unopposed estrogen exposure of either endogenous or exogenous origin. Obesity, nulliparity, and late menopause appear to be associated with high endogenous levels of unopposed estrogen. In obese women, there is an increased peripheral conversion of androstenedione to estrone by fat cells. Nulliparity seems to be associated with endometrial cancer because ovarian dysfunction (chronic anovulatory cycles and polycystic ovaries) contributes to both the infertility and the unopposed estrogen levels. Estrogen-secreting tumors, such as granulosa cell tumors, are associated with endometrial cancer up to 25% of the time. Other risk factors include a history of pelvic irradiation, a history of breast or ovarian cancer, and use of tamoxifen (Table 60.3).
TABLE 60.3 Factors Associated with an Increased Incidence of Endometrial Cancer | ||
|---|---|---|
|
Oral contraceptives appear to provide protection from endometrial cancer. At least eight population-based studies suggest that this is so. The use of oral contraceptives appears to decrease the risk of developing endometrial cancer in women 20 to 54 years of age by 50% over those women who never have used oral contraceptives. This protective effect appears to last for at least 10 years in women who used oral contraceptives for at least 1 year. Cigarette smoking also decreases the risk of endometrial cancer. There appears to be a dosage relationship to this benefit, such that the greater the number of cigarettes smoked the less the risk of developing cancer. In one study, this decrease was quite profound: 30% when one pack was smoked and another 30% if more than one pack was smoked. Furthermore, the greatest risk reduction was in the heaviest women. This is not too surprising, because it is these women who are at the highest risk of developing endometrial cancer, so they might be expected to enjoy the greatest benefit from a factor which decreased their risk. This benefit of risk reduction is strongly outweighed by the increased risk of other health problems of smoking such as heart and lung disease and, therefore, appears to be small consolation.
The relationship between unopposed estrogen exposure and endometrial cancer is well described. Although the risk of endometrial cancer is increased in these women, their prognosis tends to be better. Women with unopposed estrogen use tend to have lower stage, lower grade lesions with better prognosis.
Race is another predictor of survival and type of endometrial cancer. White women have a higher incidence
but also have a higher survival rate than black women. This originally was thought to be secondary to socioeconomic status, in that the diagnosis was made later because of less access to health care, and therefore, the cancer was of a higher stage. However, when these variables are controlled for, the survival appears to be less in black women than white. The exact reason for this is unclear. Black women have similar survival to white women when matched for poor prognostic factors. It appears that black women tend to have more poor prognostic variables, especially histologic type. With the advent of the Human Genome Project, a better understanding of the genetic differences among cancers can be more thoroughly evaluated and better understood. This may give us some insight into how different genetic changes occur or are differentially expressed in different patient populations.
but also have a higher survival rate than black women. This originally was thought to be secondary to socioeconomic status, in that the diagnosis was made later because of less access to health care, and therefore, the cancer was of a higher stage. However, when these variables are controlled for, the survival appears to be less in black women than white. The exact reason for this is unclear. Black women have similar survival to white women when matched for poor prognostic factors. It appears that black women tend to have more poor prognostic variables, especially histologic type. With the advent of the Human Genome Project, a better understanding of the genetic differences among cancers can be more thoroughly evaluated and better understood. This may give us some insight into how different genetic changes occur or are differentially expressed in different patient populations.
Breast cancer is the most common cancer diagnosed in women in the United States, thereby making tamoxifen a widely used drug in this country. It is estimated that about 80,000 women will start taking tamoxifen each year. Tamoxifen is a selective estrogen receptor modulator, and it has varying effects on estrogen-responsive tissue. The endometrium responds to tamoxifen stimulation much like it does to estrogen. Therefore, the effects of tamoxifen on the endometrium are like those of unopposed estrogen, so the risk of endometrial cancer in women on tamoxifen therapy is increased. In 1985, Killackey reported on three patients with breast cancer who were receiving tamoxifen and then developed endometrial cancer. This and many other reports suggest that there is a significant increase in the risk of developing endometrial cancer while on tamoxifen. The National Surgical Adjuvant Breast and Bowel Project (NSABP) is one of the best studies published on this subject. This study analyzed 2,843 patients with node-negative estrogen receptor–positive breast cancer who were randomized to placebo or 20 mg of tamoxifen a day. There was a significant increase in the risk of endometrial cancer in the tamoxifen arm such that the relative risk was almost three times that of the control arm. These data combined with others suggest that the increased risk of endometrial cancer in women taking tamoxifen is between two and three times that of the population not taking tamoxifen. Women who have breast cancer are also at increased risk to develop endometrial cancer. These studies often do not take this increased susceptibility into account and therefore may overstate the risk of endometrial cancer in women taking tamoxifen. The benefits of tamoxifen use for prevention of breast cancer recurrence, more than 120 per 1,000 women, far outweigh the risks of 6 endometrial cancers per 1,000 women.
Genetics
Cancer is the result of accumulation of mutations within the human genome. These mutations can be either acquired or inherited. As more becomes understood through the Human Genome Project and other research focused on more subtle DNA differences, there will be even better understanding of the mechanism by which these cancers develop. It is therefore essential that clinicians be aware of the information provided by the Human Genome Project as well as other genetic research and use it to better help their patients.
Endometrial cancer is the most commonly inherited gynecologic cancer. The clearest hereditary link to this disease is seen in HNPCC, also known as the Lynch syndrome after Henry Lynch, who helped to define this familial cancer syndrome. Clinical features of HNPCC were described first in the medical literature in 1913 by Aldred Warthin, who identified a clustering of predominantly stomach and endometrial cancers in the family of his seamstress. This inherited pattern of cancers gained little attention over the subsequent 50 years or more until characterized by Henry Lynch as a cancer family syndrome. Subsequently, the pattern of autosomal dominantly inherited risk of gastrointestinal cancers and gynecologic cancers became known as HNPCC, although some experts prefer to use Lynch syndrome because they think that it more accurately describes the disease that encompasses both the colorectal tumors and extracolonic cancers.
HNPCC is a clinically defined disease in which the primary genetic defect has been identified as a mutation in the mismatch repair genes. It is an autosomal dominant disease with a varying risk of penetrance. This is usually around 80% for the commonly mutated mismatch repair genes MLH1 and MSH2. The less commonly inherited mutations may have a penetrance that is somewhat less. That means that about 80% of the patients who inherit one of these mutations will develop a cancer. During the 1990s, specific genes responsible for HNPCC were identified and are known as the mismatch repair genes. Assigning an individual the diagnosis of Lynch syndrome prior to the discovery of these genes was based only on family history, and presently, the diagnosis can be assigned to those who meet diagnostic criteria (Amsterdam or Bethesda) as well as if an inherited genetic mutation in one of the mismatch repair genes is identified. The clinical criteria for defining this disease can be seen in Tables 60.4 and 60.5. These criteria have evolved gradually from those factors focused on colorectal cancers to those that may encompass other cancers associated with the syndrome. Not all patients who fit the criteria of having clinical HNPCC have a mutation in any of these mismatch repair genes, indicating that there is still more to learn about the development of this disease. It is believed that as many as 10% to 30% of cancers ultimately may be categorized as familial. Most of the inherited abnormalities in this group of patients are unknown. Only about 5% of all endometrial cancers can be characterized as HNPCC or Lynch. Thus, the disease of interest, HNPCC, accounts for an important, but minor, percentage of all colorectal or endometrial cancers. It is now becoming understood that women with relatives with this disease can be at increased
risk for other malignancies. This brings home the point that all women with cancers should have a detailed family history taken, which will assess their risk of developing cancers and determine any relative risk for their family members. For instance, the normal population has about a 2% chance of developing a colon cancer versus 80% cumulative risk in a patient with clear HNPCC. When counseling patients, the cumulative risk of developing a cancer over the course of one’s life is the most informative means of conveying this information. An example of cumulative risk assessment for various HNPCC cancers in a patient with HNPCC can be seen in Table 60.6.
risk for other malignancies. This brings home the point that all women with cancers should have a detailed family history taken, which will assess their risk of developing cancers and determine any relative risk for their family members. For instance, the normal population has about a 2% chance of developing a colon cancer versus 80% cumulative risk in a patient with clear HNPCC. When counseling patients, the cumulative risk of developing a cancer over the course of one’s life is the most informative means of conveying this information. An example of cumulative risk assessment for various HNPCC cancers in a patient with HNPCC can be seen in Table 60.6.
TABLE 60.4 Amsterdam Criteria | ||
|---|---|---|
|
TABLE 60.5 Bethesda Criteria | ||
|---|---|---|
|
Interestingly, the risk of a woman with HNPCC developing colorectal cancer may be less than that of a male with HNPCC (60% vs. 80%). Estrogen may play a protective role in this decreased risk. As stated previously, endometrial cancer is the second most common disease in women with HNPCC. An HNPCC mutation confers a significant cumulative risk of developing an endometrial cancer. In the normal population, a lifetime risk is about 1.5% versus 60% if one has inherited a mismatch repair defect or has clinically defined HNPCC.
Genes responsible for the development of a cancer are of two types: (a) oncogenes, which are dominantly expressed cellular control genes, and (b) tumor suppressor genes, which are nondominant cellular control genes. A more recently described set of genes is called mismatch repair genes and can be classified as tumor suppressor genes. These genes are responsible for ensuring fidelity in the DNA replicative process. The job of these genes is to search for and identify mismatches that occur during replication and to excise and repair the errors. In humans, they consist of the genes MLH1, MSH2, MSH6, PMS1, and PMS2. It is these genes that are responsible for the development of HNPCC cancers. When they are mutated and do not repair mismatches in the DNA, errors accumulate through normal division, and eventually enough errors occur in a gene(s) responsible for control of cell growth resulting in the development of a cancer. The importance of these mismatch repair genes is made clear when we see how highly conserved they are across species. The mismatch repair genes of Escherichia coli are not that different from the mismatch repair genes that are found in humans. It is now possible to test for mutations in these genes, therefore, allowing assessment of the risk of an individual getting a cancer and understanding the risk of that individual passing that risk on to offspring. Any patient with endometrial cancer should have a thorough family history taken to detect the possibility of an inherited problem. If genetic testing is deemed appropriate, this may be offered to the patient and family. However, before genetic testing is recommended, the patient should undergo counseling by a trained genetic counselor.
In summary, HNPCC is a clinically defined disease described by the Amsterdam and Bethesda criteria that has a genetic basis. The genes responsible for this syndrome can be sequenced and mutations detected. Risk of developing an inherited cancer can then be assigned to these patients. Over time, the focus on these syndromes has shifted to include extracolonic cancers such as endometrial cancer. The Amsterdam Criteria II begin to take this into account, and the Bethesda criteria continued to expand on this
concept. Importantly, gynecologists see a fair number of synchronous or metachronous ovarian and endometrial cancers. These often occur in younger women and should raise the suspicion of an HNPCC association. Any patient with cancer should have a detailed family history taken to assess her risk of developing another cancer and to help assess her family members’ risk of developing a cancer. If appropriate, family members should be offered genetic counseling and possible testing for mutation in the mismatch repair system.
concept. Importantly, gynecologists see a fair number of synchronous or metachronous ovarian and endometrial cancers. These often occur in younger women and should raise the suspicion of an HNPCC association. Any patient with cancer should have a detailed family history taken to assess her risk of developing another cancer and to help assess her family members’ risk of developing a cancer. If appropriate, family members should be offered genetic counseling and possible testing for mutation in the mismatch repair system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree