Epidemiology, Pathophysiology, and Evaluation of Pelvic Organ Support
John O. L. DeLancey
Pelvic organ prolapse is a condition that has been known to affect women since the earliest medical records 4,500 years ago. Attempts to correct this condition helped to define the specialty of obstetrics and gynecology. Although it is often discussed as a purely mechanical phenomenon, prolapse is associated with significant functional problems. Stress urinary incontinence, micturition difficulties, and problems with defecation are all associated with prolapse. These functional derangements are not simply results of altered support of the bladder and rectum but have to do with the innervation and musculature of the urinary and intestinal tracts as well. This chapter reviews the structural and functional aspects of prolapse necessary to understand and manage these conditions and describes current clinical evaluation of women who have pelvic organ prolapse.
The Pelvic Floor and the Nature of Genital Prolapse
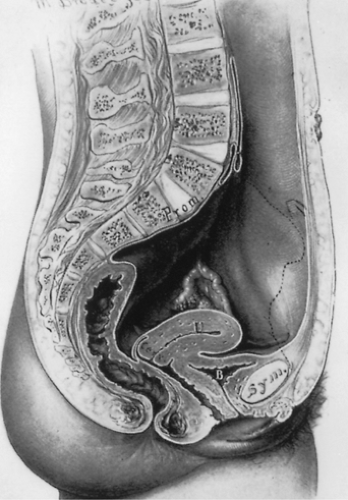
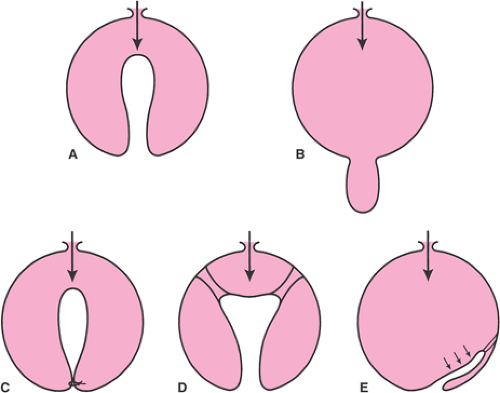
The pelvis lies at the bottom of the abdominopelvic cavity, and the pelvic floor closes the canal within the bony pelvis (Fig. 48.1). If the body cavity were a box containing the abdominal and pelvic organs, the pelvic floor would form the bottom of the box. It is the structure that carries the load. Its structural role can best be appreciated by considering a surgeon’s hand placed through an abdominal incision that pushes caudally on the pelvic organs. All of the elements that prevent this hand from passing through the pelvic canal constitute the pelvic floor. In addition to this supportive role, the pelvic floor must accommodate conception and parturition while also controlling storage and evacuation of urine and feces. To understand the pelvic floor and genital prolapse, it is necessary to understand the mechanical strategies that evolution has put in place to prevent downward descent of the pelvic organs as well as the process by which genital prolapse occurs. As Victory Bonney pointed out, the phenomenon of prolapse is similar to the maneuver that a scrub nurse uses to evert the in-turned finger of a surgical glove (Fig. 48.2). Compressing the air within the glove drives the invaginated finger outward in much the same way that increases in abdominal pressure force the vagina and the uterus to prolapse. It is not the weight of the uterus that is important in the development of prolapse but rather the forces placed on the pelvic floor by increases in abdominal pressure.
Two mechanical principles explain how the pelvic floor prevents prolapse (Fig. 48.2). First, the uterus and vagina are attached to the walls of the pelvis by a series of ligaments and fascial structures that suspend the organs from the pelvic sidewalls. Second, the levator ani muscles constrict the lumina of these organs, forming an occlusive layer
on which the pelvic organs may rest. It is a combination of these two factors—suspension of the genital tract by the ligaments and fasciae and closure of the pelvic floor by the levator ani—that holds the vagina over the levator ani muscles and forms a flap-valve closure. This flap-valve mechanism is instrumental in keeping the posterior cul-de-sac closed and preventing the development of an enterocele. Failure of this mechanism is common in women. The following sections describe how often it occurs, what changes are involved, and how to evaluate women who present with complaints of prolapse.
on which the pelvic organs may rest. It is a combination of these two factors—suspension of the genital tract by the ligaments and fasciae and closure of the pelvic floor by the levator ani—that holds the vagina over the levator ani muscles and forms a flap-valve closure. This flap-valve mechanism is instrumental in keeping the posterior cul-de-sac closed and preventing the development of an enterocele. Failure of this mechanism is common in women. The following sections describe how often it occurs, what changes are involved, and how to evaluate women who present with complaints of prolapse.
Epidemiology of Surgically Managed Pelvic Organ Prolapse
Prevalence and Age of Occurrence
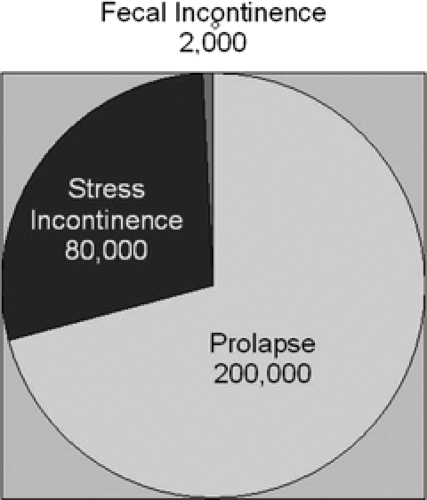
Pelvic organ prolapse is the pelvic floor disorder that most often requires surgery (Fig. 48.3), followed by surgeries for stress incontinence and fecal incontinence. Based on national hospital discharge data, it is known that approximately 200,000 American women undergo procedures for pelvic organ prolapse, while 80,000 operations per year are done for stress urinary incontinence and approximately 2,000 are for fecal incontinence. In 1997, the National Hospital Discharge Survey information indicated that this is approximately 22.7 operations per 10,000 women. The mean age of these women is in their mid-50s. The annual direct cost of treating pelvic organ prolapse is slightly in excess of $1 billion annually.
The effect of age on pelvic organ prolapse surgery has been studied in health maintenance organization populations. Olsen found that among 149,554 women over the
age of 25 who were members of Kaiser Permanente Northwest Health Maintenance Organization, 384 women had surgical treatment for either pelvic organ prolapse or urinary incontinence or both in 1 year. They documented that a woman’s lifetime risk for needing a single operation by age 80 was 11.1%. Among this group of women, there was a great variety in the types and sizes of prolapse (Table 48.1). Repeat operations were remarkably common occurring in 29.2% of patients.
age of 25 who were members of Kaiser Permanente Northwest Health Maintenance Organization, 384 women had surgical treatment for either pelvic organ prolapse or urinary incontinence or both in 1 year. They documented that a woman’s lifetime risk for needing a single operation by age 80 was 11.1%. Among this group of women, there was a great variety in the types and sizes of prolapse (Table 48.1). Repeat operations were remarkably common occurring in 29.2% of patients.
In contrast to these data concerning surgical procedures required for pelvic floor dysfunction, studies of symptomatic women in the population offer a somewhat different picture. In a study of members of a group health plan (Kaiser Permanente Southern California) where 4,458 women were selected to represent the population, the prevalence of pelvic floor symptoms was as follows: pelvic organ prolapse, 7%; stress urinary incontinence, 15%; overactive bladder, 13%; and anal incontinence (including flatal incontinence), 25%. Overall, 37% of these women had symptoms of pelvic floor disorder. The difference between the number of operations and the occurrence of symptoms relates to the severity of the problem. For example, many women have relatively mild symptoms of stress or fecal incontinence and may not consider it a particular problem and do not seek surgical correction, while more women with symptomatic prolapse have their problem addressed surgically.
TABLE 48.1 Preoperative Prolapse Severity according to Operative Site | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
TABLE 48.2 Risk Factors for Pelvic Organ Prolapse | |
|---|---|
|
Because of the increase in pelvic organ prolapse at advancing age, it is expected that the demand for services related to pelvic floor disorders can be expected to double in the next decades, justifying the need for all obstetrician and gynecologists to be experienced in its diagnosis and management.
Risk Factors
There are multiple risk factors for pelvic organ prolapse, defined as being definite, probable, or hypothesized (Table 48.2).
Factors Associated with Definite Occurrence
Age
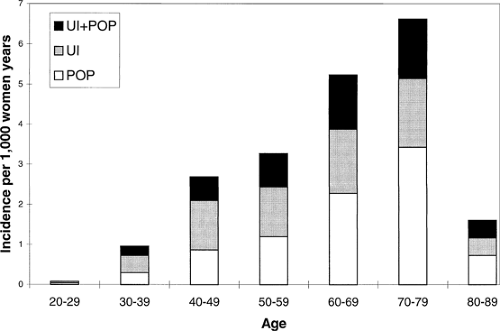
The role of advancing age in the increased occurrence of pelvic organ prolapse is obvious (Fig. 48.4). Although prolapse can occur in young women and women soon after childbirth, the number of women treated for pelvic organ prolapse increases with advancing years. Because the available information comes from counts of surgical procedures performed, there is a decrease in data for very elderly, perhaps because surgery is less likely to be performed in these women who have in increased operative risk.
Vaginal Delivery
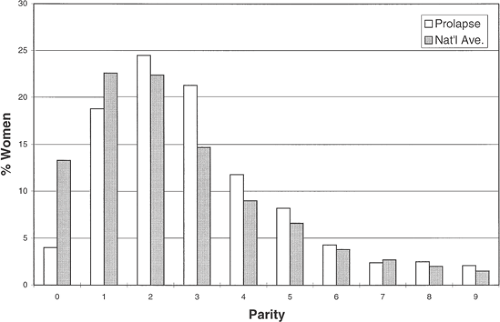
Of the pelvic floor disorders, pelvic organ prolapse is the one that is most strongly associated with vaginal delivery. In studying a longitudinally followed cohort of women, the Oxford Family Planning Study reported that there was increasing relative risk for developing prolapse with increasing vaginal parity, as shown in Figure 48.5. Although the likelihood of developing stress incontinence is also related to parity, this association is weaker (relative risk 2.4) than that of prolapse and advanced parity. The strength of this relationship depends to some extent on how one defines
prolapse. This strong association is found for women who require surgery.
prolapse. This strong association is found for women who require surgery.
Specific features of vaginal birth also influence whether or not a woman develops prolapse later in life. Several factors that can be grouped together as descriptors of “difficult” vaginal delivery are associated with increased occurrence of prolapse: forceps delivery, prolonged second stage of labor, and large infant birth weight have been associated with pelvic organ prolapse. Unfortunately, because of the overlapping nature of these different factors, it is difficult to determine which are causal and which are associated. Forceps delivery is often used when there has been a prolonged second stage of labor, and both of these factors are increased in infants of large size.
The role of childbirth in causing damage to the levator ani muscle, which is both associated with vaginal delivery and with pelvic organ prolapse, is probably the mediating mechanism in these injuries. This will be discussed later in the chapter in somewhat more detail.
Factors Associated Probable Occurrence
Heritable Issues
Although there are incontrovertible data concerning the relationship between advancing age and vaginal delivery in causing pelvic organ prolapse, there are a number of factors that have supportive evidence indicating their relationship to increased risk for developing pelvic organ prolapse but for which data are less well established. For example, some data suggest that race may play a role in modulating the likelihood that a woman may develop prolapse. In the 1997 National Discharge Summary, the surgery rate for whites (19.6 in 10,000) is approximately three times greater than it is for blacks (6.4 in 10,000). Other published data have indicated that Hispanic and Asian women appear to have an increased risk of developing pelvic organ prolapse when compared with white individuals, with modest increased risks of 1.20 among Hispanics and 2.20 in Asian women and decreased risk of 0.63 for black women.
Prolapse seems to also occur more often in some families than others. Women who report that their mother had pelvic organ prolapse have an odds ratio of 3.0 for having prolapse compared with those without a family history, and women with a sister who have prolapse have an odds ratio of 2.4. This is likely related to heritable changes in pelvic floor tissues. There is information that women with prolapse have a decrease in type I collagen and an increase in type 3 collagen compared with women who do not have prolapse. In addition, circumstantial evidence suggests that patients with Marfan syndrome or Ehlers-Danlos syndrome may have an increase in prolapse occurrence. However, the overall number of women with these syndromes who have prolapse is only a tiny fraction of women presenting with this prolapse. Further evidence of genetic factors is emerging from new investigations of elastin homeostasis in knock out mice.
Increased Abdominal Pressure
The structural supports of the pelvic organs are subjected to the forces created by gravity and increases in abdominal pressure. There is evidence that chronic or significant increases in abdominal pressure may be related to increased occurrence of prolapse. Women who have pelvic organ prolapse are more likely to report straining at stool as a young adult than women without prolapse, and women with stage II or greater pelvic organ prolapse are also known to have an increased risk of reporting constipation compared with women who do not have prolapse. Because difficult defecation can be a symptom of prolapse, it is somewhat difficult to prove that it is causal. In addition, women who do more physically stressful occupations have a threefold odds ratio for developing prolapse compared with professional or managerial women.
Hypothesized Factors for Occurrence
Several other factors that increase the risk of prolapse have been suggested, although they are less well established. Obesity, for example, seems to have a modest increase in risk for having prolapse; however, these studies have not had substantial number of patients with clinically evident prolapse (that below the hymeneal ring). Case-control information about women with definite prolapse and definite normal support has not found a difference in body mass index (BMI). There are several authors who have called attention to differences in shape and orientation of the bony pelvis between patients with prolapse and nonprolapse controls. Ironically, these seem to have to do with the upper dimensions of the pelvis, so the biomechanical reason for this is less well known.
There has long been speculation that a previous hysterectomy might alter the chance that women will develop pelvic organ prolapse. In the Oxford Family Planning cohort, women who had previously undergone hysterectomy developed prolapse at a rate of 29 in 1,000 woman-years versus 16 in 1,000 woman-years for the entire cohort. However, the gap between previous hysterectomy and prolapse is quite long, being approximately 20 years, so there must be caution in making implications about the relationship between hysterectomy and prolapse. In addition, prolapse may in some ways be part of an indication for surgery although not listed in a way that retrospective review can discern, thereby potentially confounding these analyses.
A number of other factors such as young age at first delivery, pregnancy in the absence of vaginal delivery, and selective estrogen receptor modulators have also been suggested as potentially influencing prolapse, yet definitive data concerning these issues at the present time validated by independent studies has not been available.
Anatomy and Pathophysiology of the Pelvic Floor
Connective Tissue Supports
Anterior and Apical Anatomy
The topmost layer of the pelvic floor is a combination of the pelvic viscera and their connections to the pelvic walls and will be referred to as the viscerofascial layer. Although it is common to speak of the fasciae and ligaments as separate from the pelvic organs, unless these fibrous structures have something to attach to (e.g., the pelvic organs), they have no structural integrity of the support unit.
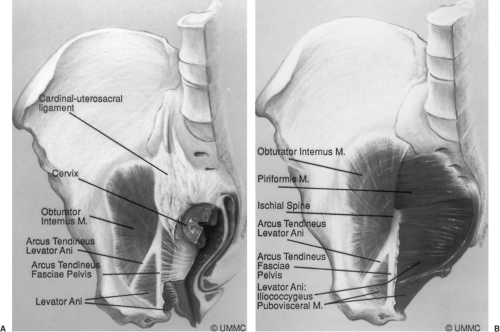
The uterus and vagina are attached to the pelvic walls by the fibrous tissue referred to as the endopelvic fascia. It forms a sheetlike mesentery that is continuous from the uterine artery to the point at which the vagina fuses with the levator ani muscles as it passes through the urogenital hiatus. The parametria are tissues that connect the uterus, and the paracolpium are those that attach to the vagina. Although given regional names, they are one continuous entity. The parametria comprise the cardinal and uterosacral ligaments. These are two different elements of the same tissue (Fig. 48.6). The uterosacral ligaments are the visible and palpable medial margin of the cardinal–uterosacral ligament complex. As is true of the remainder of the parametria, they contain smooth muscle, nerves, and blood vessels and are not the same type of tissue seen in the fascia of the rectus abdominus muscle, which is dense regular connective tissue.
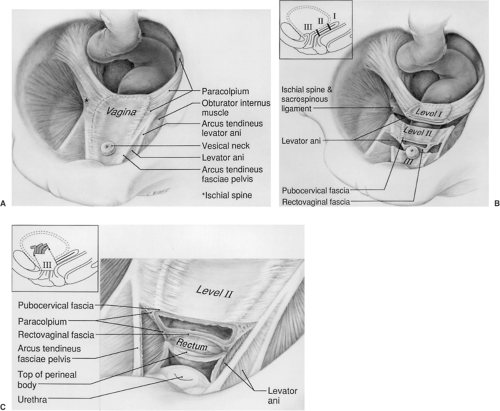
Opposite the external cervical os, the sheet of tissue that attaches the genital tract to the pelvic wall arbitrarily changes name from the parametrium to the paracolpium. The paracolpium has two portions (Fig. 48.7). The upper portion (i.e., level I) consists of a relatively long sheet of tissue that suspends the vagina by attaching it to the pelvic wall in an area similar to that of the cardinal–uterosacral ligament complex. It is this portion that prevents the upper vagina from prolapsing after the uterus has been removed.
In the midportion of the vagina, the paracolpium attaches the vagina laterally and more directly to the pelvic walls (i.e., level II). This stretches the vagina transversely between these two lateral attachments (Fig. 48.7B). This arrangement has functional significance. The structural layer that supports the bladder (i.e., pubocervical fascia) is composed of the anterior vaginal wall and its attachment through the endopelvic fascia to the pelvic wall. The term fascia is used commonly, but this is not a layer separate from the vagina. Fibromuscular layer of the vagina, which
contains both smooth muscle and connective tissue, is the term that has been proposed for this layer. These terms are used interchangeably in the remainder of the text because the surgeon generally refers to this layer as fascia. Similarly, the posterior vaginal fibromuscular layer and its connection to the pelvic walls form the restraining layer that prevents the rectum from protruding forward. In the distal vagina (i.e., level III), the vaginal wall is attached directly to surrounding structures without any intervening paracolpium.
contains both smooth muscle and connective tissue, is the term that has been proposed for this layer. These terms are used interchangeably in the remainder of the text because the surgeon generally refers to this layer as fascia. Similarly, the posterior vaginal fibromuscular layer and its connection to the pelvic walls form the restraining layer that prevents the rectum from protruding forward. In the distal vagina (i.e., level III), the vaginal wall is attached directly to surrounding structures without any intervening paracolpium.
The support that lies under the urethra has special importance for urinary incontinence. The endopelvic fascia in this region is better developed and is tougher than the tissues of the upper vagina in the area under the bladder. This provides better support for the vesical neck than for the bladder. This layer of suburethral endopelvic fascia attaches laterally to the arcus tendineus fasciae pelvis and also to the medial border of the levator ani muscles. Loss of this normal support of the urethra at the vesical neck is responsible for stress incontinence of urine.
Mechanism of Anterior/Apical Support
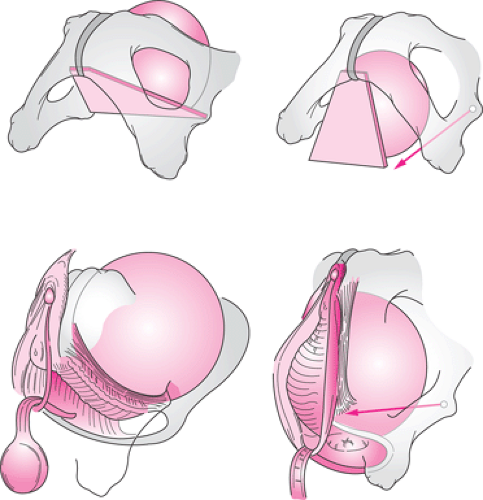
Structural support of the upper vaginal wall and uterus is intimately related. Loss of anterior vaginal wall support and uterine descent typically are part of the same process. This can be seen in the strong correlation between the degree of anterior vaginal wall/bladder descent (cystocele) and the degree of apical descent present in women. An understanding of the structural mechanics of that interaction
can be seen in Figure 48.8. From a conceptual standpoint, the bladder can be considered to rest on a trapezoidal-shaped region of the anterior vaginal wall, as seen in the diagram. The lateral margins of this trapezoid lie at the arcus tendineus fascia pelvis, with the top held in place by the apical supports and the bottom attached at the pubic bones.
can be seen in Figure 48.8. From a conceptual standpoint, the bladder can be considered to rest on a trapezoidal-shaped region of the anterior vaginal wall, as seen in the diagram. The lateral margins of this trapezoid lie at the arcus tendineus fascia pelvis, with the top held in place by the apical supports and the bottom attached at the pubic bones.
With loss of apical support, this supportive layer rotates downward with prolapse of the bladder. This leads to a separation between the sidewall structures at the arcus tendineus and the edge of the pubocervical fascia in what has been referred to as a paravaginal defect. At present, it is not known whether this process directly involves detachment from the ischial spine or failure of the cardinal uterosacral complex or both.
Data indicating the strength of this relationship is presented in Figure 48.9A. They represent measurements taken from magnetic resonance imaging (MRI) scans made at maximal Valsalva. The most dependent point of the bladder and the location of the uterine cervix were marked in women with varying degrees of pelvic organ support, ranging from normal support to prolapse. The correlation between bladder descent and descent of the cervix is strong, with half of the variation in bladder descent being explained by descent of the apex. Increased anterior vaginal length is responsible for an additional 30% of cystocele size with other less well-understood factors responsible for the remaining contribution to anterior wall descent.
Posterior Support Anatomy
The anatomical structures involved in posterior vaginal wall support are shown in Figure 48.10. The upper portion of the posterior vaginal wall is suspended by the dorsal component of the cardinal–uterosacral ligament complex. The posterior arcus tendineus fascia pelvis can be seen to extend from this upper margin to the perineal body below. It is the connective tissue at the lateral vaginal wall and is not a separate structure from the vagina itself. Distally, the vagina and arcus fuse with structures of the perineal body.
The perineal body unites the perineal membrane from one side of the pelvis with the other side (Fig. 48.11). It is the connection of the two perineal membranes in the midline that provides structural continuity to this supportive apparatus. As long as the connection is intact, it can resist downward descent of the perineum. If this connection is broken (Fig. 48.11B), then this structural continuity is lost. The anal sphincter complex closes the rectum.
Figure 48.12 shows a simplified version of the structural mechanics of this region. Although it does not capture all of the intricacies of this support system, it provides a useful depiction of the major elements in posterior vaginal wall support. In panel A, the borders of the posterior compartment are shown with the perineal body and anal sphincter at the bottom, the levator plate formed by the decussation of the levator muscles in the midline dorsally, and the posterior vaginal wall on the ventral side. The levator muscles are responsible for holding the posterior compartment in the normal location where the posterior vaginal wall is held against the anterior wall (panel C). In panel B, it can be seen what happens when the levators do not adequately close the pelvic floor. In this instance, high pressures within the rectum are not counterbalanced by contact with the anterior vaginal wall, and the connective tissue supports must come into play in order to resist this downward descent.
Pathophysiology of Posterior Compartment Problems
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree