Ultrasound in Obstetrics
Santosh Pandipati
John C. Hobbins
Ultrasound has evolved dramatically over the last 35 years and has gone from a tool used to answer a limited number of clinical questions to an essential tool in the care and management of virtually every modern pregnancy. The technology has evolved from the generation of limited, static two-dimensional (2D) images to real-time moving three-dimensional (3D) images. In addition, the practitioner now has the ability to “fly” through any acquired volume to better appreciate fetal anatomy—a technological breakthrough that has never been possible before. Indeed, so extensive and penetrating has the ultrasound revolution been that this chapter, in its finite scope, can only serve as a brief summary of much of modern obstetrical practice.
Early Pregnancy
Early pregnancy can be divided into the preembryonic period (conception to 5 menstrual weeks); the embryonic period, during which time organogenesis is the major activity (4 to 9 menstrual weeks); and the early developmental or fetal period, during which time the fetus continues to grow.
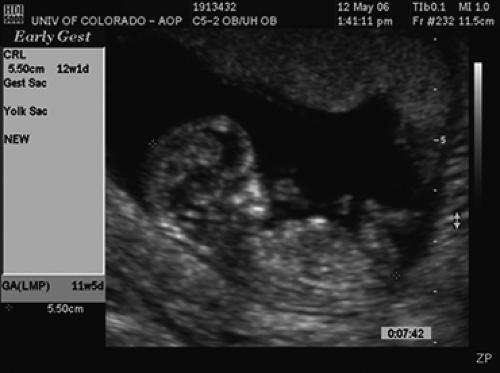
The first ultrasound sign of pregnancy is a gestational sac that appears as a double ring that is comprised of the decidua capsularis and the decidua parietalis. The sac should be seen when the β human chorionic gonadotropin (hCG) is between 1,000 and 2,000 mIU/mL. Once identified, the sac diameter should grow by an average of 1 mm per day.
The yolk sac can be seen when the mean sac diameter (MSD) is 5 mm, and it definitely should be seen by the time the MSD is 8 mm. The yolk sac provides nourishment and produces the stem cells that develop into red blood cells, white blood cells, and platelets. Thus, until approximately 7 menstrual weeks, the yolk sac provides the immunologic potential for the fetus, after which time those functions are taken over by the fetal liver.
By 5 menstrual weeks, one can see an embryo. One can determine gestational age by adding 42 days to the crown-rump length (CRL) measurement in millimeters (Fig. 9.1). The embryo should increase its CRL by 1 mm per day. Failure to visualize an embryo when the MSD has reached 6 mm is indicative of a pregnancy loss.
Cardiac activity should be visualized when the embryonic length is 4 mm or greater, and the absence of a heartbeat at this embryonic size is an ominous sign. Additionally, it has been noted that with a heart rate (HR) less than 90 beats per minute (BPM) in pregnancies that are less than 8 weeks, there is an 80% chance of fetal death. If the HR is below 70, virtually 100% will ultimately experience an intrauterine demise.
Human Chorionic Gonadotropin
hCG is a product of the human placenta that rises linearly throughout the first trimester and decreases through the second trimester. The assays commonly used today for monitoring early pregnancy measure intact hCG.
Initially, Kadar and colleagues described a “discriminatory level,” above which one should see an embryo sonographically. These initial values were based on transabdominal ultrasound (TAU) and an assay that has been replaced by the second international standard. The hCG level above which one should identify an embryo by transvaginal ultrasonography (TVU) is now 1,000 to 2,000 mIU/mL, as determined by the second international standard. When there is clinical concern for a potential pregnancy loss or an ectopic pregnancy, serial measurements of hCG can be useful. In a normal intrauterine pregnancy, the hCG level
generally doubles every 48 hours and at minimum should increase by more than 66% in that time period.
generally doubles every 48 hours and at minimum should increase by more than 66% in that time period.
The Natural Progression of Early Pregnancy Loss
A great number of pregnancies are lost within days of conception. Thereafter, the loss rate diminishes steeply until the 12th week of gestation. A patient can be counseled with regard to the chances of a losing a pregnancy based on the following sonographic findings.
|
Loss rates increase in the setting of first-trimester bleeding. It has been estimated that about 25% of all patients will have some bleeding or spotting in the first trimester, and in half of these pregnancies, a viable fetus will not result. The most common reason for early loss is aneuploidy. Ohno found that 69.4% of products of conception from 144 spontaneous abortions had abnormal chromosomes, with the majority representing trisomies. Also, the overwhelming majority of pregnancies are nonviable many days before the onset of vaginal bleeding, and the embryonic size can provide information as to when demise might have occurred.
Examination of the Placenta
The placenta is a fetal organ, and many fetal problems are linked in some way to the placenta. Indeed, even early maternal complications, such as preeclampsia, can be directly traced to the placenta.
Placental Position
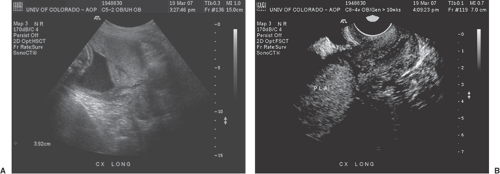
Determining placental location is a requirement of every set of guidelines for a basic ultrasound examination. The main clinical concerns regarding placental location are whether the placenta obstructs the cervix or if it is located anteriorly in the lower uterine segment over a prior cesarean section scar.
The incidence of placenta previa (Fig. 9.2) in full-term pregnancies is about 2.8/1000. However, this incidence rises with increasing parity, approaching 5% in patients with five or more previous pregnancies. The rate of placenta previa is higher in women of advanced maternal age (AMA), in those with multiple gestations, and in those
having had previous cesarean sections. With the current U.S. cesarean section rate of 29%, there is an increase in the prevalence of placenta previa, and with it, an increase in associated complications such as preterm birth (PTB) and placenta accreta is expected.
having had previous cesarean sections. With the current U.S. cesarean section rate of 29%, there is an increase in the prevalence of placenta previa, and with it, an increase in associated complications such as preterm birth (PTB) and placenta accreta is expected.
A placenta that is within 2 cm of the internal os of the cervix is unlikely to remain low lying. About 5% of patients will have a placenta previa diagnosed between 10 and 20 weeks, but only 10% of these will remain over or close to the endocervix at term. However, if the diagnosis is made at 28 to 31 weeks, 62% will persist, and if found between 32 and 35 weeks, about 75% will remain at delivery.
In placenta previa, the extent to which the placenta overlaps the cervix appears to be extremely important. Studies show that if the placenta extends past the cervix by 1.5 cm in the second trimester, the likelihood of placenta previa at term is about 20%. If the overlap is more than 2.4 cm, then 40% of these will remain.
This apparent migration of the placenta is due to passive movement away from the cervix as the lower uterine segment expands with advancing gestational age.
The transvaginal examination with the bladder empty is the best way to diagnose placenta previa, although the combination of TAU and TVU may be needed to identify an accessory lobe or interconnecting vessels to an accessory lobe (i.e., a vasa previa).
Placenta Accreta
Placenta accreta occurs in about 1 in 10 patients with placenta previa, compared with 4 per 10,000 in the general pregnant population. The risk for this condition is elevated in patients of AMA with previa who have also had a previous cesarean section. In this circumstance, there is a 40% chance of placenta accreta. Additional clinical clues as to the presence of an accreta is an elevated maternal serum α-fetoprotein (MSAFP) that is significantly greater than 2.5 multiples of the median (MoM); such an abnormality in the MSAFP has been noted in half of patients with placenta accreta.
The strongest ultrasound markers for placenta accreta are the findings of placental lakes, often beneath a previous cesarean section scar, that possess a characteristic, slow lacunar flow. It is difficult to outline an uninterrupted myometrial margin as evidenced by a sonolucent clear space even when there is no accreta, and thus this is not necessarily a reliable marker. The diagnosis is easier to make when there is invasion through the myometrium (increta) or through the serosa and potentially into adjacent organs, such as the bladder (percreta). It is important to not mistake dilated basal veins for the tornado-shaped lacunar areas synonymous with accreta. The former will have a clear rim of myometrium under them.
Magnetic resonance imaging (MRI) may be useful adjunctively when the evidence for placenta accreta is equivocal by sonographic exam, especially when the placenta is implanted posteriorly.
Placental Abruption
Placental separations most frequently occur in the placental periphery. The blood then travels extramembranously to the cervix and then through the cervical canal. The diagnosis can be confirmed if the blood stops along the way as a clot, but this occurs in only 50% of cases. In these cases, the diagnosis is made by excluding a placenta previa and documenting clinically that the bleeding is emanating from the cervical os and not from the cervix itself.
Rarely can the diagnosis can be made by identifying with ultrasound a separation between the placenta and the uterine wall. Additionally, in such cases, the patient or the fetus is usually showing signs of clinical instability.
The overwhelming majority of patients with one vaginal bleed will not have a recurrence. However, they do have a higher rate of PTB and premature rupture of the membranes (PROM). In the latter case, Lockwood has postulated that the presence of the components of the clot itself will have a direct effect on the integrity of the membranes. Also, with abruption, the membranes are separated from their source of nutrition for some time, making them more susceptible to rupture.
Placental Grading
In 1979, Grannum published a placental grading classification that was originally designed to replace amniocentesis for fetal pulmonary maturity. Although the most mature-grade placenta was a very reasonable predictor of pulmonic maturity, it was only found in about 15% of term pregnancies. In current clinical practice, the grading system has largely fallen out of use.
Abnormalities of the Cord
Single Umbilical Artery
A single umbilical artery (SUA) is found in 0.2% to 1.0% of pregnancies. Since it has been associated with a higher rate of fetal anomalies, it is important to be certain of the diagnosis. The best way to confirm cord vessel number is the identification of an umbilical artery on either side of the fetal bladder. A variant of the SUA theme is a segmental fusion occurring anywhere along the length of the umbilical cord, and this may be the reason for an erroneous diagnosis of a SUA being made with cross-sectional views alone.
Many studies have documented the association between SUA and congenital anomalies, and these rates vary between 33% and 74%. The major anomalies most commonly associated with SUA involve the fetal heart, central nervous
system (CNS), and kidneys, and thus these organ systems should be thoroughly evaluated. Chromosomal abnormalities linked with SUA are trisomies 13 and 18. If any fetal anomaly is found, then the risk of aneuploidy also increases appreciably and warrants an amniocentesis.
system (CNS), and kidneys, and thus these organ systems should be thoroughly evaluated. Chromosomal abnormalities linked with SUA are trisomies 13 and 18. If any fetal anomaly is found, then the risk of aneuploidy also increases appreciably and warrants an amniocentesis.
Umbilical Cord Insertion
The umbilical cord is usually inserted into the main body of the placenta, but variations can occur. The most extreme is velamentous insertion, where the cord inserts into the membranes close to the margin of the placenta. Velamentous or marginal insertions have been associated with a higher rate of intrauterine growth restriction (IUGR), especially in twin and multifetal gestations. In general, velamentous cord insertions that occur more inferiorly in the uterine cavity, and hence closer to the cervical internal os, tend to involve longer exposed vessel lengths than velamentous insertions occurring higher up in the uterus. Predictably, the incidence of variable decelerations and “nonreassuring fetal status” are significantly greater when a velamentous insertion occurs in the lower uterine segment versus higher locations. As a result, the cesarean section rate is also greater with lower-segment velamentous insertions as compared with higher insertions.
Vasa Previa
Vasa previa, a potentially lethal problem, complicates approximately 1 in 2,500 pregnancies overall and occurs in as many as 1 in 293 pregnancies conceived by assisted reproductive technology (ART). Vasa previa can occur either when the connecting vessels from the main body of the placenta course directly over the cervix to an accessory lobe or when a velamentous insertion of the cord resides in the membranes lying directly over the cervix. Presumably, since the vascular environment of the lower uterine segment is poorly suited to support placental development, the placenta preferentially grows superiorly while atrophying inferiorly, leaving the umbilical cord in the same place—over the cervix—but with no cushion of intervening placental tissue. As a result, although standard performance guidelines of a basic ultrasound examination do not include a search for the cord insertion, this should certainly be evaluated among all patients with either a low-lying placenta or a placenta previa.
The diagnosis of vasa previa can be made with color Doppler ultrasound demonstration of blood vessels traversing immediately over the endocervix. This is best done with TVU, and with pulse Doppler, the artery in question can be seen to be beating at a fetal rate.
Nuchal Cord
The inadvertent finding of an umbilical cord around the neck creates angst that in virtually every case is unnecessary. Indeed, about 1 in 5 fetuses have at least one loop of cord around the neck at delivery. In one observational study involving 11,200 deliveries, 19.0% of infants were born with one loop of cord around the neck, 5.3% had two loops, and 1.2% had three. From an ultrasound perspective, in a recent study, follow-up information was obtained from 118 consecutive fetuses diagnosed with ultrasound to have nuchal cords between 17 and 36 weeks. These data were compared with 233 matched controls. There was no difference in time of birth, cesarean section rate, abnormal fetal HR pattern, meconium-stained amniotic fluid, low Apgar scores, or admissions to the newborn special care unit.
Assessment of Amniotic Fluid
An excess of fluid, i.e., polyhydramnios, does not directly affect the fetus but can lead to preterm labor. In contrast, insufficiency of fluid (oligohydramnios) can have a negative impact on fetal lung and limb development, both of which need adequate amniotic fluid to develop.
The amniotic fluid volume rises linearly until about 33 to 34 weeks, when the average is about 1,000 cc, after which time it drops slowly to about 800 cc at 40 weeks gestation and to 600 cc at 42 weeks.
There are three sonographic methods commonly used to assess the adequacy of amniotic fluid—the vertical pocket technique, the amniotic fluid index (AFI), and subjective assessment.
The largest vertical pocket concept came into being when it was first described by Manning and Platt in 1981 as part of the biophysical profile (BPP). Two centimeters is considered the lower limit of normal for a single deepest vertical pocket. Most investigators use a pocket exceeding 8 cm to connote polyhydramnios.
The AFI was devised by Phelan in 1987. The uterus is divided into four quadrants, and the largest vertical fluid pockets in each quadrant are measured and totaled. An AFI equal to or greater than 20 cm constitutes polyhydramnios.
The single vertical pocket technique has been compared with the AFI, using a dye dilution technique by amniocentesis as a gold standard. Three studies showed that AFI had a poor correlation with amniotic fluid volume (R2 of 0.55, 0.30, and 0.24), and two of these three studies demonstrated a slightly better performance with a cutoff of either a single vertical diameter of 2 cm or two pockets of 2 cm. Also, in general, it is reasonable to find a vertical pocket that does not contain cord on standard 2D for measurement purposes.
Finally, the subjective assessment of amniotic fluid volume by an experienced operator is at least as good as any of the above attempts at over-quantification, since the aim of the exercise simply is to determine if there is too much, too little, or a reasonable amount of amniotic fluid present.
Abnormalities of Amniotic Fluid Volume
Oligohydramnios
Fetal renal abnormality, IUGR, and ruptured membranes are three of the most serious possibilities for oligohydramnios, and these should be ruled out before considering this finding to be a normal variant.
For any degree of oligohydramnios due to renal abnormalities, both kidneys would have to be affected. It is important to assess the size and texture of the kidneys as well as the configuration of the kidneys, ureters, and bladder.
In the setting of growth restriction, an affected fetus will shunt blood to the cerebral cortex at the expense of peripheral perfusion, including renal perfusion. With decreased renal blood flow, less urine is produced and oligohydramnios occurs.
Once urogenital anomalies and growth restriction have been ruled out, the remaining important diagnosis of consideration is rupture of membranes. This may be deduced from a patient’s description of a sudden onset of leakage of fluid from the vagina that does not seem to be related to urination. A pelvic examination is of course helpful to confirm the diagnosis, but on occasion, the clinical history and examination may be equivocal. Ultrasound may reveal diminished amniotic fluid volume transabdominally. A TVU examination also can be extremely helpful by identifying membranes coursing over the cervix if they are intact. In contrast, the integrity of the membranes cannot be demonstrated when oligohydramnios is secondary to ruptured membranes. This method is a better excluder of ruptured membranes than confirming rupture, because in the setting of oligohydramnios, the ability to visualize the membranes may be hampered.
In severe oligohydramnios or anhydramnios, irrespective of the cause, the major threat to the fetus is pulmonary hypoplasia, especially if the fetus is deprived of amniotic fluid during the second trimester (especially prior to 24 weeks gestation), when bronchiolar branching is in progress.
When oligohydramnios is truly isolated, it is reasonable to expectantly manage such a patient.
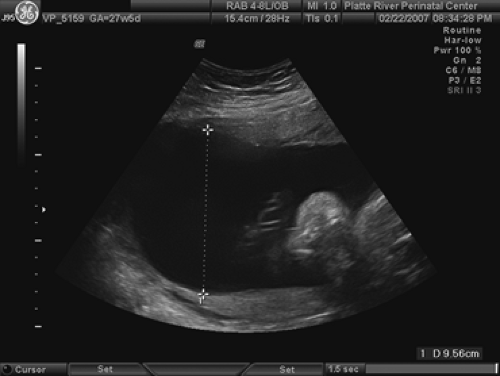
Polyhydramnios
An AFI of greater than 20 cm or a single pocket of greater than 8 cm defines polyhydramnios (Fig. 9.3), which theoretically means an amniotic fluid volume exceeding 2,000 cc. This happens in 0.25% to 3% of pregnancies. When present, the etiologies can include a gastrointestinal (GI) obstruction above the ileum, a tracheoesophageal fistula, a CNS abnormality, or a syndrome that would interfere with fetal swallowing (including the presence of a mass causing shifting or compression of the mediastinum that can obstruct the esophagus). In addition, the incidence of aneuploidy can be as high as 20% in polyhydramnios. Carlson and Platt noted the increase in fetal anomalies to be mostly in those with AFIs of greater than 24 cm. Thus, a careful evaluation of the fetal heart, GI tract, CNS, and limbs is necessary. If an abnormality is found, karyotyping should be undertaken.
Poorly controlled maternal diabetes can be associated with polyhydramnios. The mechanism appears to be fetal polyuria secondary to fetal hyperglycemia. Indeed, fetal macrosomia, with or without diabetes, can result in excess amniotic fluid volume due to a substantial but proportional production of urine. A patient presenting with polyhydramnios, a large fetus with body-to-head disproportion, and a large fetal bladder should be investigated for diabetes.
In severe polyhydramnios, therapeutic amniocentesis may be required for patient comfort or to discourage the development of preterm labor. Certainly, karyotyping would be worthwhile once the fluid has been obtained.
Fetal Measurements
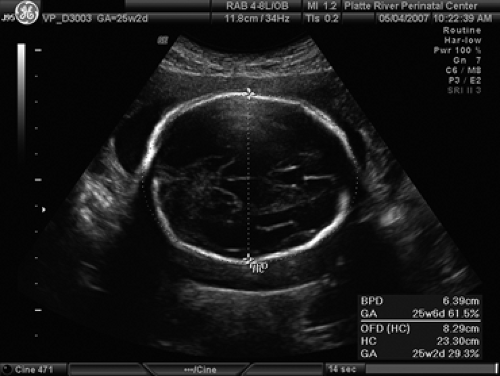
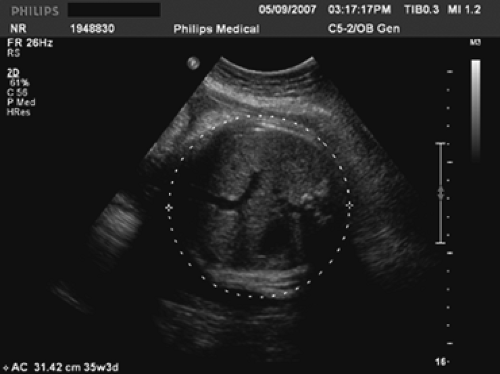
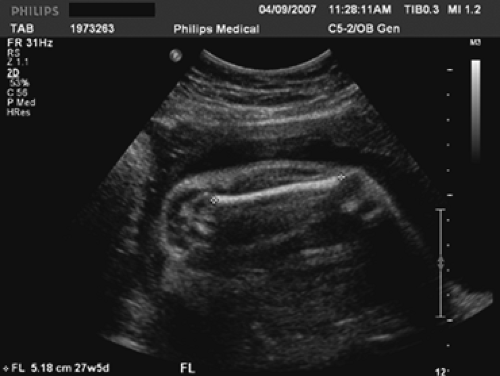
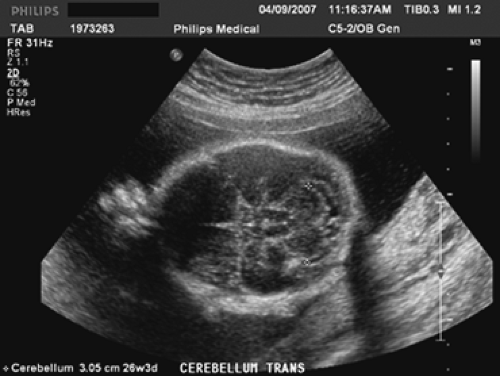
The fetal measurements generally accomplished during a standard ultrasound examination of the fetus are the biparietal diameter (BPD) (Fig. 9.4), the head circumference (HC) (Fig. 9.4), the abdominal circumference (AC) (Fig. 9.5), and the femur length (FL) (Fig. 9.6). Although each measurement tells us something different about the fetus, the relationship between these measurements will give an observer an idea regarding the fetal configuration and will often represent familial tendencies toward small or large stature. Also, the trancerebellar diameter (TCD) (Fig. 9.7) is an excellent indicator of gestational age, especially where alterations of growth and development exist.
Estimated Fetal Weight
There are more than 40 formulas in the literature for estimated fetal weight (EFW). The Hadlock formula, which
is incorporated into most North American ultrasound machine software packages, utilizes four variables: the BPD, HC, AC, and FL.
is incorporated into most North American ultrasound machine software packages, utilizes four variables: the BPD, HC, AC, and FL.
The greatest problem with EFW is not the accuracy of the formula used but the growth curves into which the EFWs are plotted. Most North American machines have Hadlock’s growth curve, which was constructed from a mixed population at sea level in Houston, Texas. Other growth curves available are from the East and West coasts of the United States as well as from countries in Europe and Asia. In Denver, Colorado, at 5,000 feet above sea level, EFWs are about 5% lower than the EFWs from Houston, translating into Denver’s 15th percentile being analogous to Houston’s 10th percentile. To compound the confusion, growth curves in the literature are not only from different populations but also are based on a variety of EFW formulas.
Part of the difficulty is that EFW is based on diameters and circumferences that are simply reflecting the volume of the fetus, but there is no reflection of fetal density in such calculations.
Despite all of these limitations to EFW, it does allow the clinician to quantify a deficit and to evaluate for the presence or absence of adequate growth over a given time interval. In addition, it enables some level of patient counseling by neonatologists regarding the prognosis for a premature fetus.
Estimating Gestational Age
Gestational age is commonly determined by taking the average of the biometric parameters (BPD, HC, AC, FL). This is usually displayed on the ultrasound report page as an average ultrasound age (AUA). This can then be compared with the patient’s menstrual dates. The dating precision varies according to the gestational age of the patient. A first-trimester CRL is the most accurate way to date a pregnancy, but
Chervenak has found that the BPD in the second trimester also has very reasonable accuracy. However, most clinicians will use the AUA to establish dates. The TCD is a very good indicator of gestational age in the second trimester, and it is the best dater of pregnancy in the third trimester because it rarely is affected by aberrations in fetal growth.
Chervenak has found that the BPD in the second trimester also has very reasonable accuracy. However, most clinicians will use the AUA to establish dates. The TCD is a very good indicator of gestational age in the second trimester, and it is the best dater of pregnancy in the third trimester because it rarely is affected by aberrations in fetal growth.