Ectopic Pregnancy
Beata E. Seeber
Kurt T. Barnhart
Ectopic pregnancy, the implantation of a fertilized ovum outside of the endometrial cavity, is a condition that is unique to primates. Although ectopic pregnancy remains a leading cause of life-threatening first-trimester morbidity, informed clinical suspicion and modern diagnostic procedures now routinely lead to diagnosis and treatment at the early signs of symptoms. Management of ectopic pregnancy has changed dramatically over the years. Medical therapy with systemic methotrexate, an intervention targeted specifically toward proliferating trophoblasts, is now often preferred to surgery as standard first-line treatment. However, surgery remains the first choice when rupture causes intraperitoneal hemorrhage, medical failures, neglected cases, and cases where medical therapy is contraindicated. In the wake of these changes, the United States has seen a considerable drop in maternal morbidity and mortality from this disease.
Early diagnosis and selection of optimal therapy are key to prevention of complications, preservation of fertility, control of costs, and elimination of mortality. The optimal dosing protocol for methotrexate therapy remains controversial. Similarly, the timing and technique for surgical intervention during medical failures are for the most part empirical. Using an evidence-based approach to the diagnosis of and treatment for ectopic pregnancy, this chapter provides a comprehensive examination of the standard of care for this serious gynecologic disease.
Incidence
The incidence of ectopic pregnancy in the United States is not known precisely. Recent attempts by the Centers for Disease Control and Prevention (CDC) to estimate the incidence of this disease have been thwarted, because there are no clear reporting standards and many cases are treated medically in outpatient facilities and are thus not recorded in hospital registries. The latest reported numbers date back to the mid 1990s. Where hospital records were used, a relentless increase in ectopic pregnancies from 4.5 per 1,000 in 1970 to 16.8 per 1,000 in 1989 to 19.7 per 1,000 (108,000 cases) in 1992 was reported. Several recent epidemiologic trends make it likely that the current incidence of ectopic pregnancy is even higher. First, there is a continued increase in the risk factors associated with ectopic pregnancy (Table 5.1). Second, there is increased ascertainment of ectopics from use of more sensitive and specific diagnostic methods that detect many cases that in the past may have resolved spontaneously without diagnosis or treatment (increase in prevalence due to lead-time bias). Third, with the increasing use of assisted reproductive technology (ART) for treatment of infertility, there is increased risk of ectopics, which comprise up to 5% of pregnancies achieved by using ART. Not surprisingly, heterotopics also are being reported with increasing frequency in ART pregnancies. Between 1979 and 1986, 13% of maternal deaths were secondary to ectopic pregnancy; by 1992, this dropped to 9%. However, ectopic pregnancies continue to be the leading cause of maternal death in the first trimester, accounting for 5% to 6% of all maternal deaths in the United States. Ninety percent of these deaths were due to hemorrhagic complications.
Pathogenesis
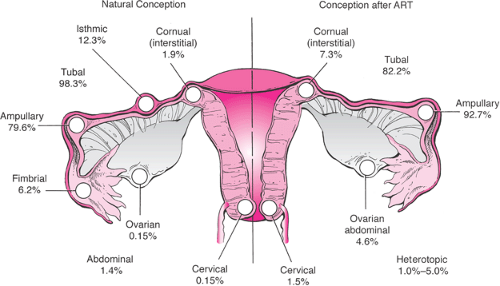
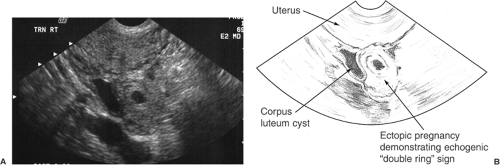
Any event that impairs the ability of the tube to transport gametes or embryos will predispose to ectopic implantation. The most common site of ectopic pregnancy is the fallopian tube, which accounts for 98.3% of all ectopic gestations. Of tubal implantation sites, the ampulla is observed in 79.6%, 12.3% are in the isthmus, 6.2% are in the fimbrial end, and the remaining 1.9% occur in the interstitial (cornual) region. Ectopic nidation outside the fallopian tubes is rare; only 1.4% of ectopic pregnancies are abdominal pregnancies, 0.15% ovarian, and 0.15% cervical (Fig. 5.1).
TABLE 5.1 Risk Factors Associated with Ectopic Pregnancy | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
In most tubal implantations, the proliferating trophoblast invades the tubal wall. Ectopic pregnancies in the ampullary portion of the tube are often within the tubal lumen and have not caused tubal rupture, while those in the isthmic portion are more likely to be found outside the lumen, having caused tubal rupture. The degree of trophoblastic invasion of maternal tissues, the age and viability of the pregnancy, and the site of implantation determine the sequence of clinical events. As the trophoblasts proliferate, the growth may extend from the luminal mucosa, into the muscularis and lamina propria, through to the serosa and, ultimately, full thickness even into large blood vessels in the broad ligament. With vascular disruption, bleeding takes place that distorts the tube, stretches the serosa, and causes pain. The embryo is abnormal and degenerates in about 80% of cases. If left untreated, spontaneous tubal abortion occurs in about 50% of tubal ectopic pregnancies and may often be clinically silent. Likewise, spontaneous tubal abortion with hemorrhage can occur with bleeding that is self-limited. However, the remaining cases of ectopic pregnancy will eventually cause tubal rupture and are associated with significant and possibly life-threatening hemorrhage. As noted previously, this complication is most likely to occur in the isthmic part of the tube, which has limited distensibility. Chronic tubal rupture with extension into the broad ligament can produce a pelvic hematoma that can last for several weeks. Unruptured ectopic pregnancies can produce a chronic course, with persistently elevated β-human chorionic gonadotropin (β-hCG) levels that may last for weeks.
Besides tubal disease, factors inherent to the embryo itself may theoretically lead to premature implantation in the tube, prior to its entry into the uterine cavity. However, studies have not supported the theory that genetic or other morphologic abnormalities of the embryo cause implantation at an ectopic site, as the rate of chromosomal abnormalities in surgically excised tubal pregnancies is comparable to that expected for gestational and age-related factors.
Other molecular-level factors that may be responsible for the molecular dialog between embryo and implantation site, or cell-cell and cell-extracellular matrix interactions, are being studied for their possible role in aberrant implantations.
Other molecular-level factors that may be responsible for the molecular dialog between embryo and implantation site, or cell-cell and cell-extracellular matrix interactions, are being studied for their possible role in aberrant implantations.
Risk Factors
Ectopic pregnancy most often is associated with risk factors leading to tubal epithelial damage, which alters gamete and embryo transport. Meta-analyses identify the risk factors listed in Table 5.1 as the most influential.
Tubal Damage and Infection
Documented tubal pathology carries a 3.5-fold common adjusted odds ratio for ectopic pregnancy. Patients with a previous ectopic pregnancy are six to eight times more likely to experience another ectopic pregnancy, and 8% to 14% of patients experience more than one ectopic pregnancy. The approximate recurrent ectopic pregnancy rate is 13% after a history of one ectopic and 28% after two previous ectopics. Patients with a history of tubal surgery have a 21-fold common adjusted odds ratio of ectopic pregnancy, but it is not clear if it is the tubal disease itself or the surgery required for the disease.
Tubal pathology frequently results from pelvic infections. Patients with a history of pelvic infections, including gonorrhea, serologically confirmed chlamydia, and nonspecific pelvic inflammatory disease, have a twofold to fourfold higher risk of developing an ectopic pregnancy. The ectopic pregnancy rate is 4% in women with laparoscopically documented salpingitis, compared with 0.7% in women with normal-appearing tubes. In evaluating histologic specimens of ectopic pregnancy, microscopic evidence of salpingitis is present in 38% of cases. Recurrent episodes of pelvic infections increase the likelihood of tubal occlusions: 12.8% after one infection, 35.5% after two infections, and 75% in women with three or more infections.
Salpingitis Isthmica Nodosa
Salpingitis isthmica nodosa is a disease defined by an anatomic thickening of the proximal portion of the fallopian tubes at the junction with the uterus and is histologically characterized by multiple luminal diverticula. The etiology of this disease is not known; however, this pattern of tubal pathology increases the incidence of ectopic pregnancy by 52% in age- and race-matched controls.
Diethylstilbestrol
Prenatal exposure to diethylstilbestrol (DES) alters fallopian tubal development, resulting in absent or minimal fimbrial tissue, a small tubal os, and decreased length and caliber of the tube. This abnormal tubal anatomy is associated with a fivefold increase in the risk for ectopic pregnancy.
Cigarette Smoking
Patients who smoke cigarettes are at a slightly increased risk for ectopic pregnancy. It is difficult to conceptualize the link between ectopic pregnancy and cigarettes. Theories include impaired immunity in smokers predisposing them to pelvic infections, alterations in tubal motility, or a representation of a lifestyle associated with an increased risk of tubal infection.
Contraception
Intrauterine devices (IUDs) have been associated with ectopic pregnancy. A multicenter case-controlled study conducted by the World Health Organization in ten countries found an odds ratio of 6.4 for ectopic pregnancy in current IUD users compared with pregnant controls, whereas the odds ratio was only 0.5 when the comparison was made with nonpregnant controls. Similarly, in the Oxford Study of 17,032 contraceptive users, the proportion of unplanned pregnancies that were ectopic was higher in women using IUDs compared with women taking oral contraceptives. Thus, IUDs effectively prevent pregnancy, but if pregnancy does occur in a woman using an IUD, there is increased likelihood that the pregnancy will be ectopic.
Tubal ligation carries a similar risk for ectopic pregnancy to what is observed with current IUD use. A meta-analysis using case-controlled studies found the odds ratio for tubal sterilization to be 9.3 when compared with pregnant controls and 0.52 when compared with nonpregnant controls, a finding confirmed by two additional multicenter case-controlled trials. As with the IUD, tubal ligations effectively prevent pregnancy, but if pregnancy does occur, the suspicion for an ectopic pregnancy should be high.
Tubal sterilization by using electrocoagulation procedures are associated with higher ectopic pregnancy risk than other methods of tubal sterilization, possibly resulting from tubal recanalization or uteroperitoneal fistula formation. Uteroperitoneal fistulas have been found in up to 75% of hysterectomy specimens from women with previous tubal ligations in which the tubes were cauterized flush with the uterus.
Oral contraceptives are associated with a reduced risk of ectopic pregnancy when compared with nonpregnant controls but with elevated risk when compared with pregnant controls. This protection is presumably due to the suppression of ovulation by oral contraceptives. It is therefore not surprising that patients who take emergency contraception, such as oral contraceptives after fertilization, are at
substantial risk for an ectopic pregnancy. This has been attributed to altered tubal motility, but this etiology remains controversial.
substantial risk for an ectopic pregnancy. This has been attributed to altered tubal motility, but this etiology remains controversial.
Barrier contraception (condoms, spermicides, and diaphragms) also reduces the odds ratio of ectopic pregnancy. An additional advantage may be attributed to the decreased risk of sexually transmitted diseases in women using barrier methods.
Evidence-Based Recommendation
Women with a previous ectopic pregnancy, tubal surgery, tubal pathology, or with prenatal DES exposure are at high risk for ectopic pregnancy. Women who have experienced genital infections, infertility, or more than one sexual partner have a moderate risk of ectopic pregnancy. Previous pelvic or abdominal surgery, smoking, vaginal douching, or an early age of first sexual intercourse have only a slightly increased risk of ectopic pregnancy.
Contraception, if used properly, is an effective way of reducing pregnancy, both intrauterine and ectopic. If pregnancy occurs in women with an IUD, after tubal ligation, or following emergency contraception, suspicion for ectopic pregnancy should be high. (Strength of recommendation: A.)
Signs and Symptoms
The classic symptoms of an ectopic pregnancy are abdominal or pelvic pain and vaginal bleeding or spotting in the context of a positive pregnancy test. However, these symptoms may be variable, range from mild to severe, and are neither sensitive nor specific for the diagnosis of ectopic pregnancy. Today, many ectopic pregnancies never produce symptoms; rather, they are diagnosed and treated in a timely fashion because the patient is identified as high risk. Table 5.1 summarizes and weighs risk factors that should be examined in every woman who has just been identified as being pregnant. However, the medical and economic benefits of screening asymptomatic women, including those who are considered at high risk, are outweighed by the still overall low incidence of ectopic pregnancy and the high false-positive rate of doing so. Thus, universal screening of all women, including some considered at higher risk, is not recommended. Since at least 40% to 50% of patients with proven ectopic pregnancies have no risk factors, absence of these factors is not wholly reassuring and does not exclude an ectopic pregnancy. Unfortunately, early diagnosis is not always achievable, and fallopian tube rupture secondary to ectopic pregnancy remains a relatively frequent clinical occurrence.
The most common signs are detected on abdominal examination. Abdominal tenderness is present in 90% of patients and rebound tenderness in 70%. The pelvic examination is usually nonspecific; cervical motion tenderness is present in up to two thirds of patients, while a tender adnexal mass is present in 10% to 50%. Pain radiating to the shoulder, syncope, and shock, as a result of hemoperitoneum, occur in up to 20% of patients and are indications for immediate surgical intervention.
Diagnosis
Ectopic pregnancy can be diagnosed as early as 4.5 weeks gestation. Unfortunately, visualizing an ectopic pregnancy this early frequently is not possible. More importantly, traditional laparoscopic visualization (Figs. 5.2, 5.3, 5.5) is now rarely necessary. Routine diagnostic tests are serial measurements of β-hCG, ultrasonography, uterine sampling via manual vacuum extraction or curettage, and, in some instances, serum progesterone levels.
Outpatient diagnosis of ectopic pregnancy by using various algorithms has been shown to be safe and effective without need for hospitalization even when the diagnosis is equivocal. The clinical algorithm in Figure 5.4 is highly efficacious in diagnosing ectopic pregnancy.
The diagnosis of ectopic pregnancy begins by excluding a normal intrauterine pregnancy. Transvaginal ultrasound examination should identify an intrauterine pregnancy with nearly 100% accuracy for gestations greater that 5½ weeks by identifying structures such as a gestational sac, a yolk sac, and fetal pole with later cardiac motion (usually seen around 6 weeks). Because of the inaccuracies inherent in pregnancy dating, β-hCG is often used as a surrogate marker for pregnancy dating. As will be further discussed below, an intrauterine pregnancy should be visualized at the “discriminatory cutoff” of β-hCG, a level corresponding to 1,500 to 2,500 IU/L (depending on
operator and equipment used) with near 100% sensitivity. These β-hCG thresholds are not universal, and each institution must identify its own values to avoid terminating normal intrauterine pregnancies. The absence of such implies an abnormal gestation. If the pregnancy is earlier than the aforementioned 5½ weeks and/or the β-hCG is below the “discriminatory cutoff,” then serial β-hCG measurements aid in the diagnosis and determine the need for intervention.
operator and equipment used) with near 100% sensitivity. These β-hCG thresholds are not universal, and each institution must identify its own values to avoid terminating normal intrauterine pregnancies. The absence of such implies an abnormal gestation. If the pregnancy is earlier than the aforementioned 5½ weeks and/or the β-hCG is below the “discriminatory cutoff,” then serial β-hCG measurements aid in the diagnosis and determine the need for intervention.
Serial β-Human Chorionic Gonadotropin Determinations
The advent of radioimmunoassay (RIA) and specific antiserum to the β-subunit of hCG has allowed for the accurate quantification of β-hCG and the ability to closely follow trends in the rise and fall of this hormone, detecting low β-hCG concentrations in urine and serum, 20 mIU/mL down to 1 mIU/mL, respectively. Currently, β-hCG is almost exclusively assayed using the third International Reference Preparation (IRP), a standard very similar to the original first IRP.
The β-hCG, produced by trophoblastic cells in normal pregnancy, has long been accepted to rise at least 66% and up to twofold every 2 days. Recent data has shown that the minimum rise for a potentially viable pregnancy that presents with pain and/or vaginal bleeding may be as low as 53% in 2 days, based on the 99th percentile confidence interval (CI) around the mean of the curve of normal
β-hCG rise. Thus, intervention for a β-hCG rise of less than 66% over 2 days, a practice supported by previous data, may potentially interrupt a normally developing intrauterine pregnancy. This generally applies to β-hCG values below 10,000 mIU/mL.
β-hCG rise. Thus, intervention for a β-hCG rise of less than 66% over 2 days, a practice supported by previous data, may potentially interrupt a normally developing intrauterine pregnancy. This generally applies to β-hCG values below 10,000 mIU/mL.
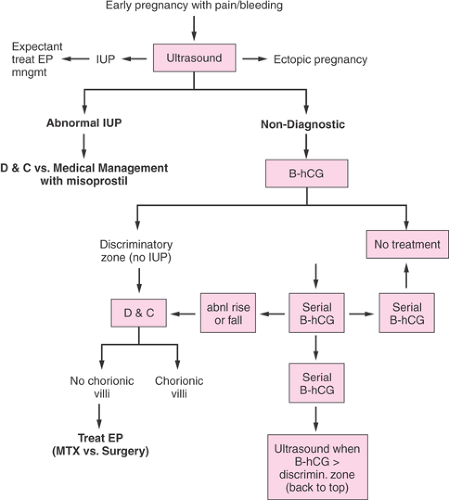 Figure 5.4 Diagnostic algorithm for ectopic pregnancy. (From Seeber BE, Barnhart KT. Suspected ectopic pregnancy. Obstet Gynecol 2006;107:402 .) |
Eight-five percent of abnormal pregnancies, whether intrauterine or ectopic, have impaired β-hCG production with an abnormal rate of β-hCG rise. β-hCG levels that plateau or fail to rise normally along with a low serum progesterone value should be considered nonviable. Rapidly declining β-hCG values (at least 21% to 35% in 2 days) are likely consistent with a miscarriage that may resolve spontaneously but could still represent a spontaneously resolving ectopic gestation. In such situations, β-hCG levels should be followed serially until no longer detectable, indicating complete resolution of the pregnancy, regardless of the implantation site.
If a viable intrauterine gestation is not visible by transvaginal ultrasonography when the β-hCG is above the “discriminatory cutoff,” and no fetal heartbeat can be visualized in the adnexa, uterine curettage or manual vacuum extraction can be performed. This intervention is necessary to accurately differentiate between an abnormal intrauterine gestation (spontaneous abortion) and an ectopic pregnancy. Either treatment of a nonviable intrauterine pregnancy is performed or ectopic pregnancy is diagnosed when the uterine contents fail to demonstrate presence of chorionic villi on histologic examination or the β-hCG levels do not fall appropriately postuterine evacuation.
If histologic examination is not readily available, β-hCG determinations are further employed for diagnosis after uterine curettage. If the β-hCG fails to decline by 15% after 12 to 24 hours from a level drawn immediately before surgery, the pregnancy is presumed ectopic and treatment should be initiated. To definitively confirm resolution of the pregnancy in the absence of a tissue diagnosis, β-hCG levels should be followed weekly until undetectable.
Progesterone
The diagnostic algorithm presented here does not include the measurement of serum progesterone levels, a test whose results are not immediately available to aid in diagnosis in many clinical settings. Although progesterone levels are higher in intrauterine pregnancies than in ectopic pregnancies, there is no established cutoff to use to discriminate between these two entities. A meta-analysis has shown that although low progesterone levels can identify patients at risk for ectopic pregnancy, this test alone is insufficient to diagnose ectopic pregnancy with certainty. In addition, a low progesterone level of less than 5 ng/mL can rule out a normal pregnancy with almost 100% accuracy but does not differentiate whether that pregnancy is an abnormal one in the uterus or at an ectopic site.
Ultrasonography
Although the uterus and adnexa may be evaluated by an abdominal or pelvic examination, transvaginal ultrasonography reliably detects intrauterine gestations when the β-hCG levels are between 1,500 and 2,500 mIU/mL (third IRP), or as early as 1 week after missed menses. An intrauterine gestation should almost always be visualized when the β-hCG level is greater than 2,000 mIU/mL.
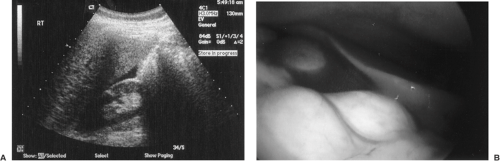
Diagnosis of an ectopic pregnancy can be made with 100% sensitivity but with low specificity (15% to 20%) if an extrauterine gestational sac containing a yolk sac or embryo is identified. A complex adnexal mass without an intrauterine pregnancy improves specificity to 21% to 84% at the expense of lower sensitivity (93.0% to 99.5%). In reviewing the literature, the presence of any noncystic, extraovarian adnexal mass in the absence of an intrauterine gestation was diagnostic of an ectopic pregnancy with 98.9% sensitivity, 96.3% positive predictive value, 84.4% specificity, and a 94.8% negative predictive value (Fig. 5.6). Despite the high resolution of transvaginal ultrasonography, an
adnexal mass will not be found in 15% to 35% of patients with an ectopic pregnancy, particularly in early stages. Some sonographic images, such as the pseudogestational sac, may mislead even an experienced examiner to falsely diagnose a gestational sac. This is a collection of fluid within the endometrial cavity, usually in a central as opposed to eccentric location that occurs due to bleeding from the decidualized endometrium when an extrauterine gestation is present.
adnexal mass will not be found in 15% to 35% of patients with an ectopic pregnancy, particularly in early stages. Some sonographic images, such as the pseudogestational sac, may mislead even an experienced examiner to falsely diagnose a gestational sac. This is a collection of fluid within the endometrial cavity, usually in a central as opposed to eccentric location that occurs due to bleeding from the decidualized endometrium when an extrauterine gestation is present.
Serial β-hCG concentrations and transvaginal ultrasonography predict ectopic pregnancy with a positive predictive value of 95%. Ultrasonography should be used to document the presence or absence of an intrauterine pregnancy when the β-hCG levels have risen above the designated discriminatory cutoff zone.
However, in those patients with an “indeterminate” ultrasound, 25% have an ectopic pregnancy. Therefore, serial β-hCG and ultrasonography alone cannot diagnose all ectopic pregnancies. In order to make the definitive diagnosis and differentiate an abnormal intrauterine from an ectopic pregnancy, uterine evacuation for tissue diagnosis is necessary. In order to minimize the inadvertent interruption of a desired intrauterine pregnancy, a high (not low) discriminatory zone should be used before uterine evacuation is considered.
Uterine Evacuation
Uterine curettage or manual vacuum extraction is necessary when a transvaginal ultrasonogram and a rising or plateauing β-hCG level below the cutoff value are not sufficient for diagnosis. With this procedure, tissue can be obtained to look for intrauterine products of conception. If present, the patient had an abnormal intrauterine pregnancy and now a completed abortion; if negative, the patient has an ectopic pregnancy needing further management. If histologic examination is not available, then a guideline of a decrease in the β-hCG level of 15% or more 12 hours after curettage is diagnostic of a complete abortion. If the β-hCG titer plateaus or rises and the trophoblast was not removed by curettage, an ectopic pregnancy is likely.
Evidence-Based Recommendation
Serial β-hCG determinations, transvaginal ultrasonography, and uterine sampling allow for definitive diagnosis of ectopic pregnancy. A confirmatory laparoscopy is rarely necessary. (Strength of recommendation: A.)
Treatment for Ectopic Pregnancy
Medical Management
Methotrexate therapy of ectopic pregnancy has been used successfully over the last 2 decades. The folic acid antagonist, methotrexate, inhibits de novo synthesis of purines and pyrimidines, interfering with DNA synthesis and cell multiplication. Rapidly proliferating trophoblasts are very dependent on folic acid and thus differentially vulnerable to the cytotoxic effect of methotrexate, and this differential sensitivity forms the basis of the therapy. When methotrexate is administered to pregnant women undergoing planned termination, a single dose of 50 mg/m2 significantly blunts the β-hCG increment over the following 7 days and has been associated with a drop in circulating progesterone and 17α-hydroxyprogesterone concentrations prior to abortion. It appears that methotrexate directly impairs trophoblastic production of hCG with a secondary decrement of corpus luteum progestin secretion. Hemodynamically stable patients with unruptured ectopic pregnancy measuring less than or equal to 4 cm by ultrasonography are eligible for methotrexate therapy. Patients with larger masses or evidence of acute intra-abdominal bleeding should undergo immediate surgical treatment. Methotrexate treatment regimens are shown in Table 5.2 and include the multiple dose, single dose, and the newly introduced two-dose protocol.
TABLE 5.2 Comparison of Methotrexate Regimens | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Multiple-Dose Methotrexate
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree