Tubal Sterilization
Herbert B. Peterson
Jeffrey S. Warshaw
Amy E. Pollack
Barbara S. Levy
DEFINITIONS
Bipolar coagulation—Method of tubal occlusion usually performed via laparoscopy that causes electrocoagulation of the tube when current is applied by grasping forceps; one jaw of the forceps is an active electrode, and the other is a return electrode. There is less chance for unintended thermal injuries than with unipolar coagulation, and the chance of unintended thermal injury by capacitive coupling is virtually eliminated. However, less widespread thermal destruction of the tube increases the chance for sterilization failure relative to unipolar coagulation unless the surgeon uses techniques that maximize the likelihood of adequate tubal coagulation.
Capacitive coupling—Unintended consequence of laparoscopic use of unipolar coagulation, which under certain conditions can result in a charge being transferred to the operative laparoscope or conductive laparoscopic sheath. Subsequent occult transference of this charge to intestines can lead to thermal injury and subsequent perforation with infectious morbidity or mortality.
Cumulative failure rates—Contrary to previous beliefs, tubal sterilization failures are not isolated to a narrow window of time after the procedure but rather increase cumulatively with each passing year following sterilization. Younger women, who are more fecund at sterilization and for years after, have higher failure rates initially and over time.
Gas embolism—Potential complication of laparoscopic abdominal insufflation with the Veress needle, associated with “vapor lock” of the right ventricle, which occludes blood flow and results in cardiovascular collapse. A “cogwheel” murmur can sometimes be heard.
Hysteroscopic sterilization—Surgical approach in which an endoscope is introduced transcervically into the uterine cavity for the purpose of placing the Essure microinsert into the fallopian tubes across the uterotubal junction. It has the advantage of requiring no abdominal incision and only minimal analgesia. It can be performed in an outpatient or office surgery setting.
Irving method—Method of partial salpingectomy that buries the end of the proximal tubal stump in the myometrium of the uterus, in an attempt to reduce the rate of tuboperitoneal fistula.
Luteal phase pregnancy—Refers to pregnancies that occurred before sterilization but were detected after sterilization. Such pregnancies may be erroneously attributed to sterilization “failure.”
Open laparoscopy—Laparoscopic procedure begun without prior insufflation by directly visualizing, elevating, and incising the layers of the abdominal wall near the umbilicus. Has the benefit of reducing vascular injuries and, potentially, bowel injuries associated with blind direct Veress needle and trocar insertion.
Parkland method—Method of partial salpingectomy that involves ligation of the tube in two places, followed by excision of the intervening segment of tube. Achieves immediate separation of severed tubal ends.
Pomeroy method—Method of partial salpingectomy that involves ligation, followed by excision, of a loop of tube. No attempt to bury the severed ends of the tube.
Poststerilization regret—Regret after the decision to undergo surgical sterilization can be related to several factors, including preexisting patient characteristics, subsequent changes in the patient’s situation or attitudes, and dissatisfaction resulting from adverse side effects caused or perceived to be caused by the procedure. Studies have identified young age at tubal sterilization as a risk factor for later regret.
Posttubal ligation syndrome—Refers to the historic perception that menstrual disturbances could result from tubal sterilization. This term is outdated, as substantial evidence against such a syndrome now exists. Women who undergo sterilization are no more likely than are their nonsterilized counterparts to experience a syndrome of menstrual abnormalities.
Silicone rubber banding—Method of tubal occlusion usually performed via laparoscopy in which a silicone rubber band is applied to the isthmic portion of the tube. The technique is most effective when the tube has normal anatomy and the operator is experienced with the technique of application. The possibility of tubal transection, and resultant hemorrhage, appears to be highest with this technique.
Sterilization timing—Sterilization can be performed in relation to pregnancy (following vaginal delivery, cesarean section, or abortion) or remote from pregnancy (interval sterilization).
Tubal clips—Method of tubal occlusion usually performed via laparoscopy in which a Filshie or Hulka clip is applied to the isthmic portion of the tube. The technique is most effective when the tube has normal anatomy and the operator is experienced with the technique of application. These methods inflict the narrowest zone of tubal damage (3 to 5 mm), affording a greater chance of sterilization reversal when compared with other methods.
Tuboperitoneal fistula—Poststerilization communication of the proximal tubal stump with the peritoneal cavity, which can result in sterilization failure, including ectopic gestation. Theoretically, may be reduced by leaving a proximal tubal stump of 2 cm and by minimizing ligature-induced necrosis at the site of tubal interruption.
Uchida method—Method of partial salpingectomy that buries the end of the proximal tubal stump within the leaves of the mesosalpinx in an attempt to reduce the rate of tuboperitoneal fistula.
Unipolar coagulation—Method of tubal occlusion usually performed via laparoscopy that causes electrocoagulation of the tube when current is applied by grasping forceps; both jaws of the forceps serve as an active electrode. The high effectiveness rates observed with this method are a property of the wide zone of thermal destruction of the tube that results, but this same property increases the potential for morbidity from unintended thermal injuries. The surgeon must be vigilant regarding possibility of capacitive coupling.
In proposing the concept of tubal sterilization in 1842, James Blundell suggested the following:
… the operator … ought to remove a portion, say one line, of the Fallopian tube, right and left, so as to intercept its caliber— the larger blood vessels being avoided. Mere divisions of the tube might be sufficient to produce sterility, but the further removal of a portion of the tube appears to be surer practice. I recommend this precaution, therefore, as an improvement of the operation.
Samuel Smith Lungren of Toledo, Ohio, is credited with having performed the first tubal sterilization in 1880, after having performed a cesarean section for a woman whose previous child was also born by cesarean section because of a contracted pelvis. During the second cesarean section, Lungren intended to remove the woman’s ovaries to prevent future pregnancy, but instead decided that “the risk would be lessened and the same result would be accomplished by tying both Fallopian tubes with strong silk ligatures a one inch from the uterus.” At the time of Lungren’s successful tubal sterilization, laparotomy was a life-threatening procedure; thus, the performance of tubal sterilization at the time of cesarean section to prevent future pregnancy was potentially lifesaving. In 1919, Madlener reported on 85 tubal sterilizations performed at the time of laparotomy for other reasons, including cesarean section; 3 of the 85 women died postoperatively from infection. Because of the extreme risks, performing a laparotomy for the sole purpose of tubal sterilization remained an unpopular idea until the mid-20th century. Indeed, when three deaths occurred from 1936 to 1950 among 1,022 women who had postpartum Pomeroy sterilization, investigators Prystowsky and Eastman concluded that the risk for sterilization was comparable to that for multiparity and that “sterilization because of great multiparity alone cannot be justified on medical grounds.”
In addition to concerns about safety, the early history of tubal sterilization included debate about the appropriateness of tubal sterilization for fertility control. At the 21st Annual Meeting of the American Gynecological Society in 1886, participants debated a woman’s right to undergo surgical sterilization. During this debate, Edward P. Davis said, “I hold it [sterilization] to be the right of a woman who is in a condition to which natural delivery is impossible….” H. J. Garrigues objected by saying,
We must leave that to Nature or to God. … I do not think that the woman has a right of that kind. … The mere fact that she does not want to have more children should not decide the question. (Speert)
The availability and acceptability of tubal sterilization as a method of fertility control remained limited until the mid-20th century, and, accordingly, tubal sterilization remained uncommon in the United States and around the world until the 1960s. In the 1970s, the worldwide popularity of tubal sterilization increased dramatically. Between 1970 and 1980, the estimated number of tubal sterilizations increased markedly in Europe, China, India, other parts of Asia, and Latin America. In the United States, the number of tubal sterilizations increased nearly fourfold—from approximately 200,000 in 1970 to approximately 700,000 in 1977. Among the factors affecting this increase were the availability and acceptability of two new surgical approaches—minilaparotomy and laparoscopy. In contrast to laparotomy for sterilization, these approaches were safer, allowed for surgery without hospitalization, reduced recovery time, and gave a better cosmetic result. Minilaparotomy has been used in many developing countries, and laparoscopy has been used in many developed countries, including in the United States.
Minilaparotomy for interval sterilization (i.e., sterilization at a time unrelated to pregnancy) requires a 2.5- to 3.0-cm suprapubic incision. The technique was first described by Uchida and colleagues in Japan in 1961. It was used in the early 1970s in Thailand by Vitoon and associates and then rapidly gained acceptance worldwide. Laparoscopy for tubal sterilization was first proposed by Anderson in 1937 and later described by Power and Barnes in 1941. The use of laparoscopy in Europe was encouraged by the work of Palmer (France), Steptoe (Britain), and Frangenheim (Germany), and use of the technique rapidly gained popularity in the 1970s, particularly in Europe and the United States.
In the United States, the increased use of tubal sterilization in the 1970s occurred concurrently with the widespread availability and acceptability of laparoscopy. In 1970, less than 1% of sterilizations were performed with a laparoscope, but by 1975, more than one third of the 550,000 women who had tubal sterilization had the procedure performed laparoscopically. This transition was associated with a marked reduction in length of hospital stay for tubal sterilization— from 6.5 nights in 1970 to 4 nights in the years 1975 to 1978. By 1987, one third of tubal sterilizations in the United States required no overnight hospital stay, and 79% of these were performed by way of laparoscopy. In 2002, the FDA approved a new even less invasive hysteroscopic approach for sterilization (Essure) that can be performed successfully in the office setting.
Sterilization is now the method of contraception most commonly used in the world. In 2009, about 251 million couples used sterilization (of themselves or their spouses) for contraception; 223 million were women using tubal sterilization and 28 million were men using vasectomy. Most of these sterilizations were performed in the developing world—229 million versus 22 million in the developed world.
In the United States, sterilization is also the most commonly used method of contraception. According to data from the National Center for Health Statistics, among women aged 15 to 44 in 2006-2010, about 14 million (22.7%) reported using either tubal sterilization (about 10 million) or vasectomy (about 4 million) for contraception. Among currently married women using contraception in 2006-2010, 47.3% were using either tubal sterilization (30.2%) or vasectomy (17.1%) for contraception. These percentages were very similar to those for 1995 (31.2% and 17.3%, respectively).
In 2006, of an estimated 643,000 tubal sterilizations, 351,000 (55%) were inpatient procedures and 292,000 (45%) were performed in an ambulatory setting. Nearly all of the former were postpartum procedures (either after vaginal delivery or concurrent with cesarean section). An increasing proportion of ambulatory procedures is being performed by hysteroscopy using the Essure device, but it is too early to determine the impact of such procedures on sterilization trends.
TIMING OF STERILIZATION
Tubal sterilization can be performed at the time of cesarean section, shortly after delivery or induced abortion, or at a time unrelated to pregnancy. About one half of tubal sterilizations in the United States are performed at a time unrelated to pregnancy. The timing of tubal sterilization can influence the choice of anesthetic, surgical approach, and method of tubal occlusion. For example, most sterilizations performed concurrently with cesarean section require no separate anesthesia and involve partial salpingectomy as the method of tubal occlusion. Most tubal sterilizations performed after vaginal delivery are done by minilaparotomy with subumbilical incisions and partial salpingectomy. Tubal sterilization not associated with birth usually is performed by laparoscopy (with use of coagulation, silicone rubber band application, or clip application), hysteroscopy (with use of Essure device), or minilaparotomy (with use of partial salpingectomy).
PREOPERATIVE EVALUATION
The candidate for sterilization should be extensively counseled. The intended permanence of the procedure, alternatives to sterilization, and risks of surgery should be discussed.
For couples desiring sterilization, no such discussion is complete without consideration of vasectomy as an alternative. Women also should be made aware that sterilization failure can occur and that the relative likelihood of ectopic pregnancy is increased when sterilization failure does occur.
For couples desiring sterilization, no such discussion is complete without consideration of vasectomy as an alternative. Women also should be made aware that sterilization failure can occur and that the relative likelihood of ectopic pregnancy is increased when sterilization failure does occur.
The workup of women who are to undergo tubal sterilization includes a history and physical examination and a laboratory evaluation, as indicated. Consideration should be given to whether the woman might be pregnant at the time of sterilization, and pregnancy testing should be ordered as necessary.
A careful gynecologic history and examination also are necessary before sterilization. Women with gynecologic disease or symptoms may require additional diagnostic or therapeutic measures. Some ultimately may be better served by other surgical procedures, either instead of or in addition to sterilization. For example, some women with enlarged and symptomatic uterine leiomyomata, women with severe dysmenorrhea, and those with symptomatic pelvic relaxation may benefit more from hysterectomy than from tubal sterilization. Others, such as women with abnormal cervical cytology, need careful evaluation before a decision can be made about preventing or treating invasive cervical cancer.
ANESTHESIA
Complications of general anesthesia are the leading cause of death attributed to sterilization in the United States. The risks inherent in general anesthesia are exacerbated by its use postpartum and during laparoscopy. The special requirements of general anesthesia for laparoscopy have been well described.
Except for the use of conduction anesthesia postpartum, general anesthesia is the technique most often used for female sterilization by laparoscopy and minilaparotomy in the United States. A 1988 survey of members of the American Association of Gynecologic Laparoscopists revealed that the number of providers of tubal sterilization who used local anesthesia for laparoscopic sterilization had increased from 4% (in the 1982 survey) to 8%. Worldwide, more than 75% of tubal sterilization procedures are performed under local anesthesia. A discussion of the technology and regimen for administering general or conduction anesthesia is beyond the scope of this chapter. Instead, we focus on local anesthesia because of its increasing use in outpatient settings for many types of surgery.
Sterilization by laparoscopy, hysteroscopy, or minilaparotomy can be performed safely under local anesthesia, and hysteroscopic sterilization with the Essure device can be performed with minimal analgesia alone. The patient avoids the risks associated with general anesthesia, spends less time sedated or anesthetized, and has a more rapid recovery. Nausea and vomiting are less likely to occur, and the patient is awake to report symptoms that can indicate the occurrence of a complication. Furthermore, the overall expense often is reduced compared with procedures done under general anesthesia.
In the United States, the overall morbidity rate for female sterilization is so low that it is difficult to obtain a sample large enough to demonstrate a comparative safety advantage for local versus general anesthesia. One US study randomly assigned 100 women to either local or general anesthesia for laparoscopic sterilization. Serious or life-threatening events did not occur in either group. However, women who had general anesthesia were more likely to have intraoperative hypotension, hypertension, or tachycardia, which suggests that these women were hemodynamically less stable and may have been at increased risk for cardiovascular complications. In another study, 125 women were randomly allocated to the use of local or general anesthesia for laparoscopic sterilization. Women who had general anesthesia were more likely to develop hypotension; hypertension was more common in the local anesthesia group. No women in either group had tachycardia.
Operating under local anesthesia incurs several possible disadvantages. The patient’s anxiety may be increased; therefore, the surgeon must use a decisive and gentle surgical technique while talking with the patient. The patient may feel discomfort; thus, the physician must have a thorough understanding of the use of sedative and analgesic drugs. Although obesity can complicate the use of local anesthesia, several studies indicate that local anesthesia can be used successfully for obese women. Women with a history of multiple abdominal or pelvic surgical procedures or peritonitis may need additional anesthesia if the procedure is difficult or prolonged. Additional anesthesia also may be required during minilaparotomy if the abdominal incision needs to be extended. The hysteroscopic approach may be ideal for both obese women and those with multiple previous abdominal procedures as it avoids the necessity to access the tubes through the abdominal wall.
One US-based retrospective study reviewed 2,827 outpatient laparoscopic sterilizations performed under local anesthesia and mild sedation from 1980 to 1988. The mean operating time was 10.0 (±5.1) minutes, and the mean anesthesia time was 23.3 (±6.9) minutes. The hospital cost to the patient was reduced 65% to 85%. Another US study reported on 358 minilaparotomies for interval sterilization performed under local anesthesia. The average operating time was 21 minutes, and no complications were reported. In both series, the local anesthetic was 0.5% bupivacaine hydrochloride used alone or in combination with lidocaine. In one series, midazolam hydrochloride and fentanyl citrate were used for mild intravenous sedation; in the other series, meperidine hydrochloride and diazepam were used.
For local infiltration and paracervical block, agents of intermediate intrinsic potency (defined as the minimum concentration required to produce a block within 5 to 10 minutes), such as lidocaine or mepivacaine, have been found suitable. Both are amides with good stability and low toxicity. Onset of analgesic effects is rapid, even when a low concentration of medication is used, and the duration of the effect is sufficient for the procedure but not prolonged (about 1.5 hours when the medication is given in plain solution). Bupivacaine, a more potent and a longer-acting amide, is frequently used in the United States. However, the short duration of action provided by lidocaine or mepivacaine is preferred by some because it allows for awareness of any abnormal degree of persistent pain and early diagnosis of complications, such as hematoma formation.
SURGICAL APPROACH
Minilaparotomy
The minilaparotomy approach to tubal occlusion can be used in the interval or postpartum period. Although interval sterilization by minilaparotomy is the sterilization procedure most frequently performed in many countries, it is not a common procedure in the United States. Minilaparotomy in the United States often is used preferentially among women considered to be at increased risk for laparoscopy.
Interval minilaparotomy is performed with the use of a 2- to 3-cm midline vertical or transverse suprapubic incision. In patients with an enlarged uterus resulting from uterine leiomyomata or other benign conditions, the minilaparotomy incision should be made at the level of the uterine fundus to ensure access to the fallopian tubes. A uterine manipulator is placed through the cervix just before surgery and is used to bring the uterus toward the incision. Placement of a paracervical block before insertion of the uterine manipulator reduces
discomfort for patients having surgery under local anesthesia. The abdomen is then entered with the approach that is used for laparotomy; small handheld retractors and the Trendelenburg position are used to enhance exposure. Once the uterus is identified, a tubal hook or a finger is placed posteriorly at the top of the fundus and moved along the uterus. The fallopian tube is identified first by the fimbriated end, and then the midportion of the fallopian tube is grasped with a small Babcock clamp and elevated through the abdominal incision. Tubal occlusion most often is performed by use of the modified Pomeroy or Parkland technique. However, clips or rings can be applied through the minilaparotomy incision with modified instruments originally developed for use through the operating laparoscope.
discomfort for patients having surgery under local anesthesia. The abdomen is then entered with the approach that is used for laparotomy; small handheld retractors and the Trendelenburg position are used to enhance exposure. Once the uterus is identified, a tubal hook or a finger is placed posteriorly at the top of the fundus and moved along the uterus. The fallopian tube is identified first by the fimbriated end, and then the midportion of the fallopian tube is grasped with a small Babcock clamp and elevated through the abdominal incision. Tubal occlusion most often is performed by use of the modified Pomeroy or Parkland technique. However, clips or rings can be applied through the minilaparotomy incision with modified instruments originally developed for use through the operating laparoscope.
Postpartum minilaparotomy is performed in a manner similar to that of interval minilaparotomy. It is ideally performed before the onset of postpartum uterine involution while the uterine fundus is high in the abdomen (within 48 hours of delivery). A 2- to 3-cm subumbilical vertical or semicircular incision is made in the midline where the abdominal wall is thin. Because of the proximity of the enlarged uterus to the incision, access to the fallopian tubes is easier than it is with an interval approach. A uterine manipulator is unnecessary when minilaparotomy is performed in the postpartum period.
Laparoscopy
The magic is in the magician, not in the wand. … Entering the abdomen is the most dangerous part of the laparoscopic procedure. (Hulka and Reich)
The laparoscopic instrumentation, including laparoscope, light cables and light source, insufflator and tubing, and video camera and television, if used, should be set up and tested for proper functioning before any incisions are made. If a Veress needle is to be used, it is good practice to attach it to the insufflation tubing and test that gas flows freely through it. High line pressures (3 mm Hg or higher) during low-flow insufflation (1 L/min) through a Veress needle that has yet to be inserted into the abdominal cavity suggest that the needle has some occlusion. Checking for occlusion before inserting the needle can avoid the multiple attempts at needle placement that might occur in the belief that incorrect placement, rather than needle occlusion, is the cause of resistance to flow. It is convenient, while waiting for surgery, to have the end of the laparoscope bathing in warm sterile fluid to prevent it from fogging when it is inserted into the abdominal cavity.
A no. 11 scalpel blade is used to make a single vertical incision in the lower rim of the umbilicus. This must be done carefully because the aorta can lie just a few centimeters beneath the abdominal wall, particularly in a thin patient. The abdominal wall is lifted away from the aorta by pinching the skin beneath the umbilicus between thumb and index finger. The umbilicus is elevated with one hand or towel clips while the surgeon’s other hand makes the controlled incision.
The Veress needle is disconnected from the insufflation tubing before insertion. The stopcock on the needle then is placed in the open position, and the spring action of the needle is tested for smooth operation. Elevating the abdominal wall and using the Veress needle with the stopcock open allows air to rush into the previously gas-free abdominal cavity when the end of the needle penetrates the peritoneal layer. The inrushing air, if heard, is one of the first indicators that successful abdominal entry has occurred. Outflow of blood likewise can serve as an immediate indicator of vessel injury. Allowing air to rush in also can cause the bowel to fall away from the abdominal wall.
The terminal aorta is palpated, even in a moderately obese patient, through the abdominal wall in the midline, just above or at the umbilicus. The pulsations of the aorta are lost in the midline just beneath the umbilicus, corresponding to the sacral hollow. The aorta bifurcates at the level of L4, which corresponds to the summits of the iliac crests. This is a more reliable landmark for bifurcation of the aorta than the umbilicus. The Veress needle is placed through the umbilical incision and is directed at an angle toward the sacral hollow or uterine fundus (to avoid the aorta) and in the midline (to avoid the iliac vessels). Elevating the abdominal wall during this placement increases the distance between the Veress needle and major vessels. While placing the Veress needle, the surgeon should hold the needle like a dart, being careful not to impede the action of the spring mechanism. Resistance at the tip of the needle causes the blunt cannula inside the needle to be pushed back, and the spring mechanism at the hub of the needle extends. When resistance at the tip of the advancing needle is lost, as occurs with successful penetration of the peritoneal cavity, the blunt cannula inside the needle advances back out to the tip, and the spring mechanism at the hub of the needle snaps back in. Observing the spring mechanism for this snap can serve as a sign to test for successful placement. Often, there are two snaps—the first when the fascia is penetrated and the second with peritoneal penetration. Continuing to advance the needle tip much beyond the point of penetration of the parietal peritoneum risks placing the needle tip between loops of bowel or under the omental apron, both of which can cause resistance to flow and can result in reinsertion of the needle. Continued advancement of the Veress needle also risks vascular injury. The needle tip should not be moved laterally once the tip is inserted in the abdomen; any simple vascular or intestinal puncture could be transformed into a major laceration if the needle tip is moved from side to side. Once abdominal penetration is made with the Veress needle, the needle should be stabilized carefully until the safety checks have been completed. The needle should not be moved, and a laparotomy should be performed immediately if major vascular injury is suspected.
The distance between skin and fascia can increase with increasing patient obesity. A given angle of entry of the Veress needle that successfully penetrates the peritoneal cavity in a thin patient might fall far short of the peritoneum in an obese patient. To bring the peritoneal cavity within the physical length of the Veress needle, it is often necessary to pursue entry with a trajectory closer to the vertical. However, this also directs the Veress needle toward the aorta and therefore should be attempted only by experienced surgeons, and with extreme care. Alternatively, open laparoscopy can be performed.
Several safety checks for correct intraabdominal placement can be performed while the Veress needle is held steady. The instillation of 5 to 10 mL of sterile saline through the needle, followed immediately by aspiration, can be helpful. The fluid should meet little resistance, and, more important, scant fluid should return on reaspiration. Reaspiration of fluid suggests that the needle tip is in a small enclosed space that does not allow immediate dispersion of the instilled fluid. Reaspiration of feculent fluid or bloody fluid suggests bowel or vascular penetration, respectively. Simple penetration of the bowel with a Veress needle does not mandate immediate exploratory laparotomy. Depending on the setting and the skill level of the surgeon, reinsertion of the needle or open laparoscopy, followed by laparoscopic visualization of the intestine, can be pursued.
A second safety check consists of attaching a filled 10-mL syringe to the hub of the Veress needle and then removing
the plunger. Elevation of the abdominal wall at this point should cause an increased negative intraperitoneal pressure, which allows the fluid in the syringe to drain passively into the abdominal cavity through an open stopcock. Alternatively, a drop of saline (drip test) can be placed on the hub of the Veress needle, and, with the stopcock open, elevation of the abdominal wall should result in the drop flowing downward freely.
the plunger. Elevation of the abdominal wall at this point should cause an increased negative intraperitoneal pressure, which allows the fluid in the syringe to drain passively into the abdominal cavity through an open stopcock. Alternatively, a drop of saline (drip test) can be placed on the hub of the Veress needle, and, with the stopcock open, elevation of the abdominal wall should result in the drop flowing downward freely.
Once these safety checks are performed, insufflation is begun at 1 L/min or less. Initial insufflation pressures often are 5 mm Hg or less in thin patients and, even with correct needle placement, can be 10 mm Hg or more in obese patients. An intraabdominal pressure greater than 15 mm Hg during insufflation generally is avoided to prevent respiratory compromise and decreased venous return secondary to vena caval compression. Abdominal distention increases the distance between the abdominal wall and major pelvic vessels, and this increases the safety buffer when the trocar is inserted. However, the abdomen should not be distended so taut that it is difficult to manually elevate it for trocar insertion.
During insufflation, a shift from dullness to tympany with percussion over the liver is indicative of pneumoperitoneum formation. It also is prudent for the surgeon to pay attention to the electrocardiogram rhythm during insufflation, because the sudden appearance of premature ventricular contractions can be an indicator of intravascular insufflation. Sudden vascular collapse during insufflation can be caused by a gas embolism into the right side of the heart and, at times, this life-threatening event can be signaled by a new “cogwheel” or “mill wheel” murmur. Rapidly tilting the patient into Trendelenburg position and onto her left side and advancing a Swan-Ganz catheter into the right heart for aspiration can be lifesaving.
Once insufflation is complete, the Veress needle is removed, and the umbilical incision is widened to accommodate the trocar and sleeve. An incision that is too large can result in leakage of gas around the sleeve. An incision that is too small can restrict access. The trocar should be checked before insertion to ensure that it is sharp; a dull trocar potentially is dangerous because it requires increased force for abdominal entry.
The trocar is grasped as shown in Figure 27.1. If the surgeon’s hand is large enough, the middle finger can serve as a stopper, preventing the forward momentum following fascial puncture from carrying the trocar deep into the pelvis. The precautions for insertion of the Veress needle pertain even more to insertion of the trocar; the abdominal wall is elevated, the trocar is directed toward the hollow of the sacrum or the uterine fundus, and a midline trajectory is followed. Slow, steady pressure with a sharp trocar will permit immediate recognition when the gas-filled cavity is penetrated.
Open laparoscopy is performed without previous creation of a pneumoperitoneum. The skin beneath the umbilicus is incised sufficiently with a scalpel in either a vertical or transverse direction to adequately visualize the underlying fascia. Small S retractors are most helpful in visualization—especially in obese patients. The skin is released, and the fascia is grasped and elevated. The fascia is then incised in the midline, and the opening is stretched with a hemostat or incised to a length just sufficient to visualize the underlying peritoneum, which in turn is elevated and incised. Keeping the incision in the central portion of the umbilicus where the peritoneum and fascia are fused is the easiest method for entry in obese patients. Each angle of the fascial incision is sutured with a no. 0 absorbable stitch but not tied. The Hasson blunt cannula and sleeve are then placed through the opening of the peritoneum. The blunt cannula then is removed, and the abdomen is insufflated through the port found on the sleeve.
METHOD OF TUBAL OCCLUSION
All tubal sterilization methods rely on correct identification of the fallopian tube for success. With any of the transabdominal methods, the tube should be followed out to its fimbriated end to confirm that the correct structure has been identified.
Theoretically, the risk of tuboperitoneal fistula formation can be reduced by preserving a proximal tubal segment 1 to 2 cm in length. It is possible that the proximal tubal stump serves as a distensible reservoir for the small amount of uterine fluid that is normally forced through the interstitial portion of the tube by uterine contractions. This distensibility capacitance of the proximal stump might serve to dissipate the fluid pressure emanating from the uterus. Otherwise, this direct fluid pressure on the cut end of the tube might prevent complete closure of the tubal lumen during the healing process.
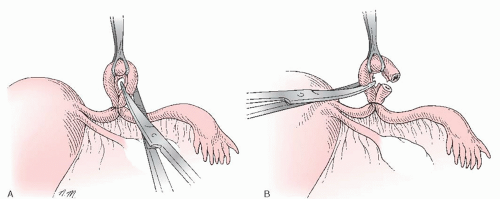
Irving Procedure
In 1924, and later in 1950, Irving reported on his method of achieving tubal sterilization. He attempted to reduce the risk of tuboperitoneal fistulae by extensively dissecting the ligated ends of the tubes and burying the proximal tubal segment. Although the extra dissection in this technique likely enhances effectiveness, it also carries the potential for greater blood loss, as well as increases the difficulty of performing the technique through a minilaparotomy incision. The procedure also takes slightly longer to perform than simpler methods.
The Irving technique is accomplished by first using a hemostat or scissors to create a window in the mesosalpinx just beneath
the tube, about 4 cm from the uterotubal junction (Fig. 27.2A). Then, the tube is twice ligated (no. 1 chromic) and divided between the ties at this location. The free ends of the proximal stump ligature are held long. A 1-cm incision is made in the serosa of the posterior uterine wall near the uterotubal junction. A hemostat, or similar pointed instrument, is then used to bluntly deepen the incision, creating a pocket in the uterine musculature about 1 to 2 cm deep (Fig. 27.2B). The two free ends of the proximal stump ligature, previously held long, are then individually threaded onto a curved needle and brought deep into the myometrial tunnel and out through the uterine serosa (Fig. 27.2C). Traction on the sutures then draws the ligated proximal stump deep into the myometrial tunnel, and tying the free sutures fixes the tube in this buried location (Fig. 27.2D). Often, this can be accomplished without incising the mesosalpinx, but if extra mobilization of the proximal stump is needed, or if the proximal stump mesosalpinx appears in danger of being torn when traction is applied, then the mesosalpinx under the proximal tubal segment can be incised partly back toward the uterus. The serosal opening of the myometrial tunnel is then plicated closed around the tube with the use of a fine absorbable suture, but great care should be exercised to avoid compromising the tube as it enters the tunnel. Strangulation or damage to the tube with this stitch could cause necrosis and fistula formation in the extramyometrial portion of the proximal tube. No treatment of the distal tubal stump is necessary, but some surgeons choose to bury that segment in the mesosalpinx.
the tube, about 4 cm from the uterotubal junction (Fig. 27.2A). Then, the tube is twice ligated (no. 1 chromic) and divided between the ties at this location. The free ends of the proximal stump ligature are held long. A 1-cm incision is made in the serosa of the posterior uterine wall near the uterotubal junction. A hemostat, or similar pointed instrument, is then used to bluntly deepen the incision, creating a pocket in the uterine musculature about 1 to 2 cm deep (Fig. 27.2B). The two free ends of the proximal stump ligature, previously held long, are then individually threaded onto a curved needle and brought deep into the myometrial tunnel and out through the uterine serosa (Fig. 27.2C). Traction on the sutures then draws the ligated proximal stump deep into the myometrial tunnel, and tying the free sutures fixes the tube in this buried location (Fig. 27.2D). Often, this can be accomplished without incising the mesosalpinx, but if extra mobilization of the proximal stump is needed, or if the proximal stump mesosalpinx appears in danger of being torn when traction is applied, then the mesosalpinx under the proximal tubal segment can be incised partly back toward the uterus. The serosal opening of the myometrial tunnel is then plicated closed around the tube with the use of a fine absorbable suture, but great care should be exercised to avoid compromising the tube as it enters the tunnel. Strangulation or damage to the tube with this stitch could cause necrosis and fistula formation in the extramyometrial portion of the proximal tube. No treatment of the distal tubal stump is necessary, but some surgeons choose to bury that segment in the mesosalpinx.
Modified Pomeroy Procedure
Bishop and Nelms, colleagues of Pomeroy, reported on the Pomeroy technique for tubal occlusion in 1930. They were careful to point out the importance of using absorbable suture as opposed to permanent suture.
In this method, the tube is grasped in its midportion, usually with a small atraumatic clamp such as the Babcock, and a loop of tube is elevated (Fig. 27.3A). The base of the loop is ligated with no. 1 plain catgut, leaving a 2-cm proximal stump of isthmus, and the sutures are held long. A 2- to 3-cm portion of tube in the ligated loop is transected and removed with scissors (Fig. 27.3B). Bishop and Nelms, in the original report on this method, pointed out that ligation was performed with a double strand of absorbable chromic catgut suture to allow the cut tubal ends to quickly separate after surgery. It was their belief that this would allow the ends to naturally fibrose and peritonealize without fistulization or communication. This also is the rationale for the common modification of the Pomeroy technique, in which the original chromic suture is replaced by plain catgut because of the more rapid degradation of the latter. Surgeons have a tendency to strenuously tighten the catgut ligature around the tube (as though the tighter the ligature, the better the occlusion), but this appears to go against the very principles of the procedure. This tightening can result in greater strangulation and necrosis of the adjoining tubal segments, potentially increasing the risk of fistula formation
and failure. Taking time to identify the muscular tube, which is often seen pouting from each of the severed limbs of the ligated tube, is a good habit to develop, as is checking the tubal stumps for hemostasis. Resection of a limited amount of tube, restricted to the isthmic section, is ideal should unexpected future reanastomosis be requested. Making the knuckle of tube in the loop too small, however, can result in only a shave excision of the side of the tube, with incomplete transection of the lumen. To avoid incomplete resection, the mesosalpinx within the ligated loop should be perforated with scissors before the tubal limb on each side of this window is individually cut. It is important not to cut the loop so close to the suture that only short distal segments of tube remain beyond the tie. These short limbs can easily slip out of the ligature and cause delayed bleeding.
and failure. Taking time to identify the muscular tube, which is often seen pouting from each of the severed limbs of the ligated tube, is a good habit to develop, as is checking the tubal stumps for hemostasis. Resection of a limited amount of tube, restricted to the isthmic section, is ideal should unexpected future reanastomosis be requested. Making the knuckle of tube in the loop too small, however, can result in only a shave excision of the side of the tube, with incomplete transection of the lumen. To avoid incomplete resection, the mesosalpinx within the ligated loop should be perforated with scissors before the tubal limb on each side of this window is individually cut. It is important not to cut the loop so close to the suture that only short distal segments of tube remain beyond the tie. These short limbs can easily slip out of the ligature and cause delayed bleeding.
The Pomeroy method minimizes bleeding by compressing and sealing the vascular mesosalpinx before tubal transection. It is not unusual when performing tubal sterilization at the time of cesarean section to find the mesosalpinx greatly engorged with distended veins. Elevation of the uterus through the abdominal incision often facilitates tubal occlusion by allowing the vessels to drain and decompress. It is important when replacing the uterus into the peritoneal cavity to lead with one adnexa at a time while protecting the tubal ligation site on that side. Otherwise, when the uterus is replaced, a tight fit can cause the adnexa to be squeezed against the incision, with resultant avulsion of the ligature and postoperative bleeding. When a Pomeroy ligation is performed through a minilaparotomy incision, the ligation sutures are held while the tube is cut. This prevents retraction of the cut tubal stumps into the peritoneal cavity before they can be adequately examined and before hemostasis can be ensured. After examination is complete, the sutures are cut, and the tubal stumps are allowed to retract into the abdomen.
It is also possible to perform a modified Pomeroy tubal ligation as a laparoscopic interval procedure. In this technique, a laparoscope with an operating channel is placed through the umbilical port, and a 5-mm midline suprapubic cannula is introduced under direct vision. A plain gut Roeder loop (endoscopic slip knot) is introduced through the 5-mm port. A grasper is introduced through the operative channel of the laparoscope, advanced through the suture loop, and the appropriate portion of tube is grasped and retracted back through the loop. The slip knot of the loop is then tightened, ligating a knuckle of the tube. Scissors are then introduced through the operative scope, and the suture is cut. With the use of a grasper through the 5-mm port, the loop of tube is held on tension while the scissors are used through the operative channel to transect the tube above the ligature. When the procedure is complete and the tubal segment has been resected, the grasper is used through the operative channel to hold the specimen while the operative scope and grasper are removed together through the umbilical sleeve. In contrast to most other methods of laparoscopic sterilization, this technique has the advantage of producing a surgical specimen for evaluation.
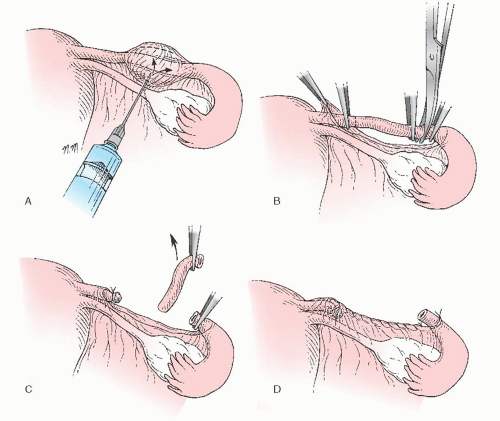
Uchida Method
Originally reported on in 1961, and reported on in revised form in 1975, the Uchida method, like the Irving procedure before it, recognized the role of fistula formation in tubal sterilization failures and included steps to prevent this complication.
This method begins by having the surgeon grasp the tube in its midportion, about 6 to 7 cm from the uterotubal junction. A 1:1,000 epinephrine in saline solution is injected subserosally, creating a bleb over the tube that is then incised (Fig. 27.4A). The muscular tube, which often can be seen springing up through the serosal incision, then is divided between two hemostats. The serosa over the proximal tubal segment is dissected bluntly toward the uterus, exposing about 5 cm of the proximal tubal segment (Fig. 27.4B). The tube then is ligated with no. 0 chromic suture near the uterotubal junction, and this 5-cm segment of exposed tube is resected. The shortened proximal stump is allowed to retract into the mesosalpinx (Fig. 27.4C). The serosa around the opening in the mesosalpinx is sutured in a purse-string fashion with a fine absorbable
stitch. Simultaneous ligation of the distal tube and gathering of the mesosalpinx around the distal stump are accomplished when the purse-string suture is tied (Fig. 27.4D). This step also fixes the distal stump in a position open to the peritoneal cavity while burying the proximal stump within the leaves of the mesosalpinx.
stitch. Simultaneous ligation of the distal tube and gathering of the mesosalpinx around the distal stump are accomplished when the purse-string suture is tied (Fig. 27.4D). This step also fixes the distal stump in a position open to the peritoneal cavity while burying the proximal stump within the leaves of the mesosalpinx.
Uchida added fimbriectomy to the procedure in 1975 to enhance effectiveness. Some surgeons omit this step, and in addition excise only 1 to 2 cm of tube (rather than the recommended 5 cm) to permit future tubal anastomosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree