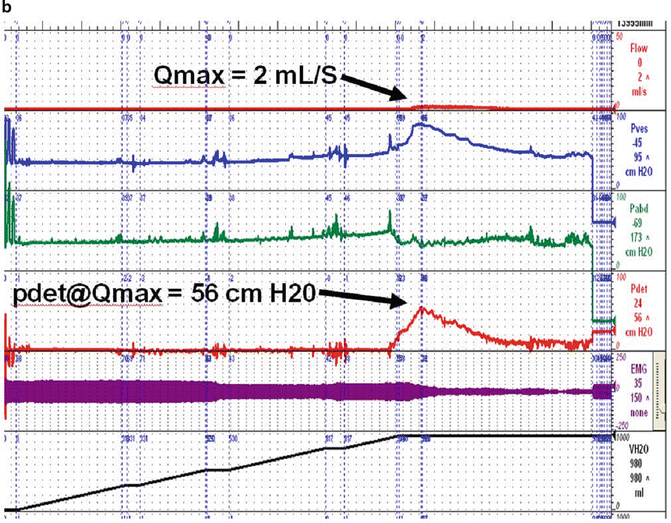
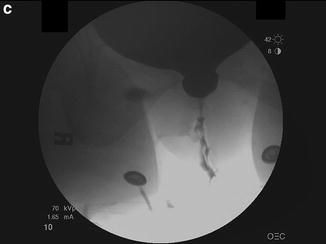
Fig. 9.1
(a) Blaivas–Groutz nomogram for bladder outlet obstruction. (b) Urodynamic tracing shows severe urethral obstruction (Type 2 in Blaivas-Groutz nomogram). Q max: maximum flow rate; P det@Q max: detrusor pressure at maximum flow rate; P ves: vesicle pressure; P abd: abdominal pressure; P det: detrusor pressure; EMG: electromyogram; V H2O: volume of water. (c) X-ray exposed at Q max shows obstruction in the distal third of the urethra. Patient underwent excision of the suburethral portion of the sling and subsequently voided normally (Q max: 19 ml/s, voided volume: 150 ml, post-void residual: 49 ml), but developed sphincteric incontinence and underwent successful autologous fascial sling 4 months later. (a: Adapted with permission from Blaivas JG, Groutz A. Bladder outlet obstruction nomogram for women with lower urinary tract symptomatology. Neurourol and Urodynam 2000;19:553; b, c: Used with permission from Blaivas JG, Purohit RS, Weinberger JM, Tsui JF, Chouhan J, Sidhu R, Saleem K. Salvage Surgery after Failed Treatment of Synthetic Mesh Sling Complications. J Urol 2013; 190:1281–1286)
Techniques of Repair
There are two basic approaches to correcting urethral obstruction after incontinence surgery—sling incision/excision and urethrolysis. Urethral stricture is a very rare complication after incontinence surgery and will be discussed in Chap. 13. No matter what technique is used, there is always a fine balance between relieving obstruction and the development of recurrent sphincteric incontinence.
Sling Incision/Excision
Simple sling incision is the least invasive and most successful procedure for women who are obstructed after sling surgery. It is indicated as a primary procedure and can be done at any time after the original surgery when a synthetic sling has been done, but is usually reserved for those who are at least a month or two after the original surgery to lessen the likelihood of recurrent sphincteric incontinence. For patients who have undergone biologic slings, we prefer to wait at least 3 months. The technique is straightforward. The patient should be placed in the dorsal lithotomy or Trendelenburg position to achieve optimal visualization of the anterior vaginal overlying the urethra [17]. A Foley catheter should be placed and by placing it on traction, it is usually possible to palpate the sling which appears as a subtle transverse ridge. If that is not successful, urethroscopy with a cystoscope or placement of a urethral sound can aide in the identification of the constricting band by torqueing upward [18]. We prefer to make a transverse incision just distal to the sling; others describe an inverted-U or midline. Some reports advocate using the prior incision or making a lateral incision to release one side of the sling, but by the time we see the patient, the site of the old incision is not apparent [19]. The incision continues down to the surface of the normal urethra distal to the sling and the dissection proceeds proximally until the sling is identified. Sometimes it is obvious, sometimes subtle, and apparent only as a thickened scar. Once identified, we place an Allis clamp on the sling in the midline and exert traction. This takes some tension off the sling and facilitates the dissection between the urethra and sling. We prefer to do the entire dissection sharply with a Metzenbaum scissor (Fig. 9.2a). If the surgical plane is obvious, it can be continued with a fine blunt instrument like a right angle clamp, but great care should be exercised so that the urethra is not damaged. In some cases the sling has been incorporated in the wall of the urethra or has eroded into the lumen. In either instance, it is necessary to excise part of the urethral wall, and we believe the entire vaginal portion of the sling should be excised as well. The defect in the urethra repaired as necessary with absorbable monofilament or chromic sutures.
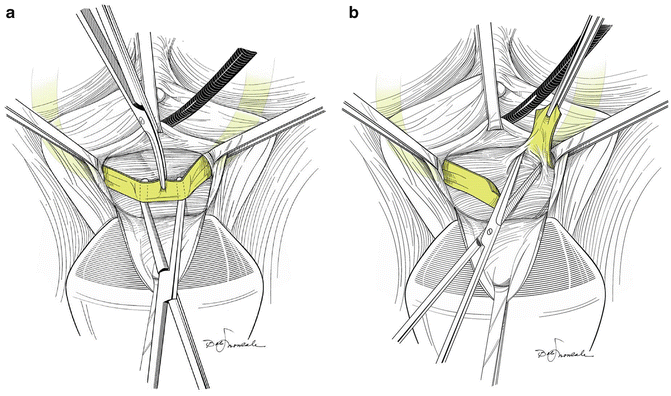

Fig. 9.2
(a) Once the sling has been dissected free of the urethra, a right angle clamp is placed between it and the urethra; the sling is transected with a scissors or knife. (b) Once the sling has been transected, it usually springs apart. If not, it should be sharply dissected off of the urethra. (Both used with permission from Nitti VW, Carlson KV, Blaivas JG, Dmochowski RR. Early results of pubovaginal sling lysis by midline sling incision. Urology 2002;59:47)
Once the sling is freed from the periurethral tissue, it is incised in the midline and the cut ends should spring apart (Fig. 9.2b). The ends of the sling material can be removed or left in place [18]. Gomelsky et al. [3] recommend that the suburethral portion of synthetic slings be excised at this point but that biologic slings be simply incised. We do not make such a distinction. If the cut edges of any type sling do not retract, we dissect them out lateral to the urethral wall on either side and then make a decision about whether or not to do more or less urethrolysis as described below.
If the sling cannot be identified by the technique described above, the dissection can be done lateral to the urethra from meatus to bladder neck if necessary and, using this method, we have always been able to identify the sling. At the conclusion of the procedure, a Foley catheter is left indwelling and in most cases, the patient is given an active voiding trial once she has fully recovered from anesthesia. The bladder is filled with saline until the patient is comfortably full, and then the catheter is removed. If she fails the trial, we would prefer to start intermittent self-catheterization, but so far, that has not been necessary in our experience. The patient is discharged home the same day [17].
Urethrolysis
Urethrolysis is indicated in women with obstruction due to periurethral scarring, usually after Burch colposuspension, Marshall–Marchetti–Krantz procedures, and in those who have undergone multiple prior urethral surgeries including sling incision and prior urethrolysis. It is rarely necessary after synthetic sling surgery unless there have been prior failed attempts at sling incision.
Urethrolysis may be performed by a variety of techniques—retropubic, transvaginal, and suprameatal. No matter what method is used, it is important to recognize that, unlike sling incision or excision, there is no clear-cut end point with urethrolysis; deciding when the dissection is complete is a matter of judgment and experience and must be individualized depending on operative findings. We begin the procedure by inserting first a Q-tip to assess urethral mobility and angle; then a Foley catheter is inserted to assess bladder neck mobility by pulling down on the catheter. As the periurethral dissection proceeds, we periodically pull down on the catheter and reinsert the Q-tip to assess the progress being made in freeing up the urethra. The goal of the surgery is to restore some, but not too much mobility. We have not found any measurements to be useful in this except that the Q-tip angle should be restored to 0 or a positive angle if it was negative to begin with.
Retropubic Approach
The retropubic approach was described in 1990 by Webster and Kreder, primarily for urethral obstruction after retropubic colposuspension or urethropexy [20]. Access to the retropubic space is gained through a low midline or Pfannensteil incision. The urethra, bladder neck, and anterior vaginal wall are freed from any adhesions with sharp dissection, including freeing of the urethra anteriorly away from the pubic bone. If there is severe scarring, the dissection can proceed laterally to the ischial tuberosities; however, this will leave a defect in the paravaginal region. An omental flap may be utilized in the space between the urethra and pubic bone to prevent future adhesions, especially in cases of recurrent obstruction after prior urethrolysis [20, 21].
Transvaginal Approach
Transvaginal urethrolysis can be performed through an inverted-U or midline incision that is made in the anterior vaginal wall over the mid urethra. A transverse incision is made over the midurethra or bladder neck as dictated by the local anatomy (Fig. 9.3a–d). The dissection is then carried proximally to the level of the bladder neck or distally to the meatus as necessary to free up the urethra. Unlike the simple sling incision, the dissection proceeds laterally, perforating the endopelvic fascia with a Metzenbaum scissors and the urethra is freed from its attachments to the vaginal sidewall and pubic symphysis as needed using blunt dissection with an index finger, with a Metzenbaum scissors, or right angle clamp. If necessary, it can be completely freed up circumferentially [3]. After circumferential urethrolysis, particularly if it is done as a tertiary procedure, a Martius graft can be mobilized and tunneled between the labia and vaginal dissection. The graft is passed anteriorly to the urethra and wrapped circumferentially around to minimize scarring and recurrent obstruction [22]. In rare instances we have completely circumscribed the entire urethra from meatus to bladder neck. If that is done, we recommend a Martius flap and autologous sling; otherwise the patient will almost assuredly have severe sphincteric incontinence [23]. A urethroscopy is performed to ensure that no urethral injury occurred.
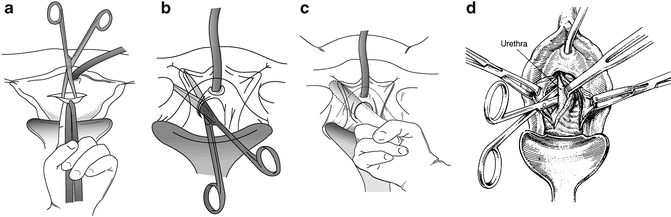

Fig. 9.3
(a) A transverse incision is made over the urethra and an Allis clamp placed on the proximal edge of the vaginal incision in the midline. With the non-dominant hand, traction is placed on the Allis clamp and the index finger holding the clamp pushes upward putting the vaginal wall on tension. A plane is dissected with a Metzenbaum scissors between the pubocervical fascia and the vaginal wall. (b) Keeping in the plane between the pubocervical fascia, with the curve of the scissors pointed laterally, the dissection proceeds in the direction of the patient’s ipsilateral shoulder, hugging the undersurface of the pubis and ileum. The technique is not spread and cut, but rather by opening and closing the tips of the scissors, and you push against the bones. The retropubic space is entered sharply with the scissors or bluntly with an index finger. (c) Once the retropubic space is entered, the urethrolysis is completed, by dissecting with an index finger medially and laterally on the undersurface of the bone. (d) If a circumferential urethrolysis is necessary, the scissor is directed medially between the pubis and urethra on either side. Once a window is created, a penrose drain is passed around the urethra and, using it as traction, the dissection can be extended proximally or distally. (a, b, c: Used with permission from Blaivas JG, Chaikin. Pubovaginal fascial sling for the treatment of all types of stress urinary incontinence: surgical technique and long-term outcome. Urol Clin North Am 2011;387–15; d: Used with permission of Shlomo Raz, MD. With permission of and from Nitti VW, Raz S: Obstruction Following Anti-Incontinence Procedures Diagnosis and Treatment with Transvaginal Urethrolysis. J Urol 1994; 152:93–98)
Suprameatal Approach
A variation on the transvaginal approach is the suprameatal technique, which begins with an inverted-U incision between the clitoris and urethral meatus (Fig. 9.4a–d). The apex of the U is anterior to the urethral meatus at the 12 o’clock position [3]. The dissection is done with a Metzenbaum scissors and it is generally easy to find a bloodless plane…for a while. At the proximal most portion of the dissection, there is sometimes a gush of bleeding which is controlled by finger pressure or by temporarily packing with a sponge. We have not found it necessary to look for individual bleeders (nor is it usually possible). It is usually possible to palpate retropubic slings and Burch sutures and to visualize them, but the space is too small to cut them under direct vision. We use a curved Mayo scissors with the curve pointed upward and cut the sling or sutures by feel. The remainder of the dissection and freeing up of the urethra is as described in the transvaginal approach. A Martius flap can also be performed in conjunction with this technique.
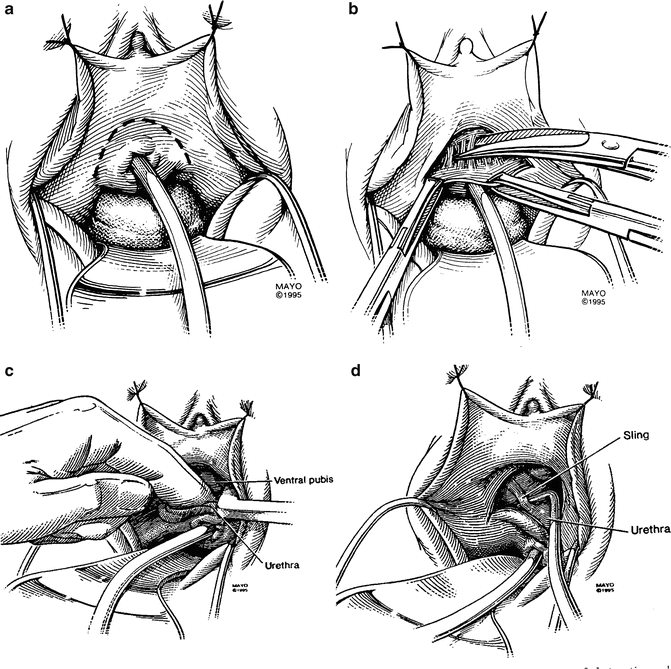

Fig. 9.4
(a) An inverted “U” incision is made 0.5–1 cm above the urethral meatus. (b).The pubo-urethral ligament is sharply taken down in the midline. (c) Finger dissection in the midline is utilized to further free up the urethra. Once the proximal surface of the pubis is reached, the retropubic space can be entered either bluntly or sharply, being careful to apply pressure in an upward direction against the bone so as not to injure the bladder neck. (d) Once the retropubic space has been entered, the sling or sutures from retropubic suspension are usually easily palpated, but may be difficult to visualize directly because of the narrow wound. It is usually possible to cut the sling or sutures blindly by palpation and cutting with the tips of the scissors pointing outward against the bone while the index finger of the non-dominant hand is pressing downward on the urethra (not pictured in this figure). (a–d: From Petrou S P, Brown J. A., Blaivas, J. G.: Suprameatal transvaginal urethrolysis. J Urol 1999; 161: 1268, 1999. Used by permission of Mayo Foundation for Medical Education and Research. All Rights Reserved)
Outcomes in the Literature
Success rates for a formal urethrolysis (retropubic, transvaginal, or suprameatal) range from 43 % to 94 % [22, 24–31]. More recently, success rates ranging from 80 % to 100 % have been described for sling incision [8, 17–19]. A review of the literature yields many retrospective studies looking at various types of surgical intervention to relieve iatrogenic obstruction following anti-incontinence procedures in women, but the number of patients is small and so many different types of primary and secondary procedures have been done that it is not possible to come to any firm conclusions except that retropubic urethropexies are much more difficult to treat successfully. Of note, the definition of success varies by study. Many categorize patients by symptom improvement, either complete or partial.
Sling Incision
Goldman et al. described a simple sling incision for iatrogenic urethral obstruction in 14 women. In this retrospective analysis, 11 women had a biologic pubovaginal sling and 3 women had a synthetic midurethral sling. With 12 months of follow-up, 11/14 had complete resolution of symptoms and 2 had significant improvement. Overall, 93 % of women had a complete or significant improvement in symptoms. One patient went on to formal urethrolysis and one underwent surgery for recurrent SUI [17]. Croak et al. reported a retrospective analysis of five women who underwent sling incision after tension-free vaginal tape (TVT) placement for SUI. All women were able to void postoperatively; however, one woman had recurrent SUI and one had persistent dysuria following urethrolysis [8]. Nitti et al. presented a retrospective analysis of 19 women with both storage and voiding symptoms who underwent lysis of a pubovaginal sling. With 12 months follow-up, 84 % had resolution or improvement of their presenting symptoms. SUI recurred in 17 % of women and three women went on to require a formal urethrolysis [18]. Kusada also described five patients who underwent incision of a pubovaginal sling for obstructive symptoms that resolved in all patients [19]. Amundsen et al. reported outcomes on a retrospective series combining sling incision and transvaginal urethrolysis. Success rates for complaints related to voiding were 94 % and 67 % for complaints related to storage [25]. These rates were not subdivided by type of urethrolysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


