Fig. 18.1
Vaginal dilators. Vaginal dilators come in variable lengths and sizes (Used with permission from Ridgeway BR, Attaran M. Embryology and Congenital Anomalies of the Urinary Tract, Rectum, and Female Genital System. In Walters MD, Karram MM eds.: Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia: Elsevier; 2014)
Physical therapy can also be used as an adjunct to vaginal dilators in the creation of the neovagina. Vaginal dilators are widely used by physical therapists for the treatment of pelvic floor disorders such as pelvic floor hypertonicity causing pelvic pain, vaginismus, vulvodynia, and dyspareunia [21]. They are used in desensitization therapy using graded exposure with a progressive increase in size of the dilator in order to treat dyspareunia [22]. Physical therapy using various heat modalities to make the tissues more pliable in conjunction with manual stretching by a therapist while the patients continues to use dilators on her own is associated with a shorter length of treatment to attain a functional vagina [23] and may be a good option for some patients and should be considered.
Surgical Management
Surgery is indicated for patients who are unsuccessful with dilators or patients who opt for surgical management after they have been thoroughly counseled about the risks and the benefits of surgery. The patient should be counseled that surgical management with vaginoplasty is not necessarily a “quick fix” and that she will need to use vaginal dilators postoperatively to maintain her surgically created vagina. Again, the goals of therapy involve the creation of a vaginal canal that is of adequate length and caliber, in the correct axis, with some secretory capacity that will allow for sexual intercourse to occur without the need for lifelong dilation. The timing of the surgery depends on the patient and the type or procedure planned. Surgeries often are performed in late adolescence when the patient is more mature and better able to adhere to postoperative dilation or instructions [24].
McIndoe Procedure
The most common surgical procedure performed in the United States to create a neovagina is the McIndoe operation, which is commonly used to treat patients with congenital absence of the vagina. The primary goal is creation of a functional vagina. The technique involves creating a canal within the connective tissue between the bladder and the rectum and using a mold to line the vagina with a split-thickness skin graft (STSG) obtained from the patient’s thigh, inguinal region, or buttocks [25] followed by progressive vaginal dilator use to achieve maximal vaginal length and caliber. In order to perform this technique, the patient is placed in dorsal supine lithotomy position. Laparoscopy may be performed first, even in cases when an intraabdominal graft is not used, in order to delineate organs such as the bladder and rectum prior to commencing the rectovesical dissection from below. Next, the vaginal dimple or foreshortened vagina is identified and a 3 cm transverse incision is made across it. Dissection is then done, using mostly blunt technique, first creating two channels on either side of the median raphe of the perineum (Fig. 18.2a). Gentle pressure is applied cephalad during dissection to create the canal with a goal depth of approximately 10–12 cm (Fig. 18.2b). Care should be taken during the dissection to avoid entry into the bladder, rectum, and posterior cul-de-sac. An EEA sizer may be used in the rectum to help with dissection. Prior to dissection, a split-thickness graft is harvested and should be approximately 10 × 20 cm in size and kept moist using normal saline during the canal dissection. The graft is passed through a skin mesher, which perforates the tissue, expanding the surface area of the graft while permitting egress of blood and fluid from the surgical site postoperatively. Once dissection is complete, the graft is placed over a dilator-like mold in an “inside-out” fashion so that the external portion of the skin lies against the mold (Fig. 18.2c). Placement should be symmetric so that the tip of the mold is at the middle of the graft with the long axis of the graft draped along the long axis of the mold on both sides. The mold is then placed inside the dissected vaginal canal and the edges of the graft are then everted and sutured in an interrupted fashion to the distal opening of the neovagina using 3-0 or 4-0 delayed absorbable suture (Fig. 18.2d, e). Patients are advised about modified or complete bed rest to avoid having the mold or stent fall out and placing tension on the distal sutures. The labia majora are often sewn together over the stent to keep it in place. A foley catheter is left in place for 5–7 days postoperatively, and is removed at the time of mold or stent removal, which is done carefully during an exam under anesthesia so as to not avulse the graft from the underlying connective tissue. Patients subsequently undergo a dilation process that can last several months to a year depending upon patient and tissue compliance.
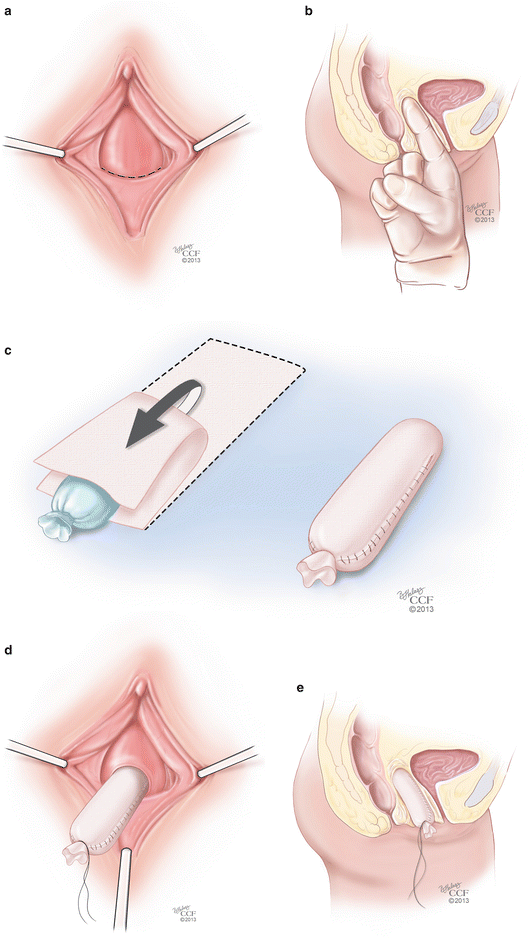

Fig. 18.2
(a–e) McIndoe procedure. (a) The initial step of the McIndoe procedure involves the identification of the vaginal dimple and the creation of a 3 cm transverse incision across it. (b) Blunt dissection can be done in the space between the rectum and the bladder in order to create the canal with a goal depth of approximately 10–12 cm. (c) Once dissection is complete, the graft is placed over a dilator-like mold in an “inside-out” fashion so that the external portion of the skin lies against the mold. (d) The mold is then placed inside the dissected vaginal canal. (e) Sagittal view of the mold inside the vaginal canal (All: Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2013. All Rights Reserved)
Studies have shown excellent results after the McIndoe operation. After 12 months, Seccia et al. [4] found that out of 32 patients, 90 % of patients presented with complete skin graft take and 84 % reported normal sexual activity with good sensitivity. The most common postoperative complications were anxiety (~6 % of patients) related to possible pain during insertion of the dilator and keloid scarring on the donor site of the skin grafts (~3 % of patients). Klingele et al. [20] looked at patient satisfaction with the McIndoe procedure and reported that 79 % believed that the procedure improved their quality of life, and 91 % were sexually active. Other potential complications reported in the literature include graft rejection, contraction of the graft, hematoma, infection, fistula formation, and excess granulation tissue [26–28].
Modifications of the McIndoe
A modification to the McIndoe procedure is the technique used to open the perineum prior to dissection. A triangular-shape inferiorly based flap approximately 3–4 cm in size can be created as the initial incision, which can then be sutured to the graft placed on the stent [29]. This method of opening can provide additional length to the neovagina, and also help create a tension-free re-approximation of the graft to the neointroitus.
A second modification to the standard McIndoe procedure is the addition of a laparoscopic intraperitoneal repair. Laparoscopically, the bladder is retrograde filled to facilitate visualization of its margins. The peritoneum is grasped at the superior edge of the bladder margin and opened and then dissected off of the underlying bladder muscularis. Dissection can be facilitated with injection of normal saline to create a hydrodissection plane. A retropubic dissection is sometimes necessary to further mobilize the peritoneum and to release the bladder from the pubic symphysis, which brings the peritoneum closer to the graft implant site. After the rectovesical dissection is complete and the mold is placed with the graft in its desired location, the peritoneal flap can be used to cover the mold. Bowel epiploica as well as the omentum, if it can be mobilized, can also be used to cover the mold.
The McIndoe procedure also has several modifications related to the type of tissue or material that is used to line the neovagina. While the split-thickness skin graft has been described as a safe and low-morbidity technique [25, 27], it has significant disadvantages. The use of a STSG can be associated with a high contracture rate, even when patients are compliant with vaginal dilation, and this can lead to inadequate vaginal length and caliber [19, 30]. Reported modifications of the McIndoe technique include replacement of the STSG with various alternatives, including autologous full-thickness skin grafts (FTSG), human amnion, peritoneum, bladder mucosal grafts, xenografts, and synthetic graft material [28, 31–33]. Techniques using FTSG are less likely to lead to neovaginal contracture and stenosis and do not require prolonged stenting [33, 34]. In addition, sebaceous and sweat glands are better preserved in these grafts, which can help with lubrication of the neovagina in some patients [35]. Akin describes a technique using a FTSG from the inner groin areas [29] (Fig. 18.3a). These grafts are used in a similar fashion to the STSG used with the traditional McIndoe procedure (Fig. 18.3b). Younger patients who require neovagina reconstruction may have limited potential graft sites for FTSG harvesting. Techniques such as tissue expander placement in sites such as the bilateral groins have been described with good outcomes and limited morbidity to the donor sites [36]. While FTSG confer many advantages when compared to STSG, the disadvantages of these grafts include skin texture mismatch and unwanted hair growth. Additionally, donor-site morbidity is slightly higher than with STSG and short-term dilation is still required with this technique.
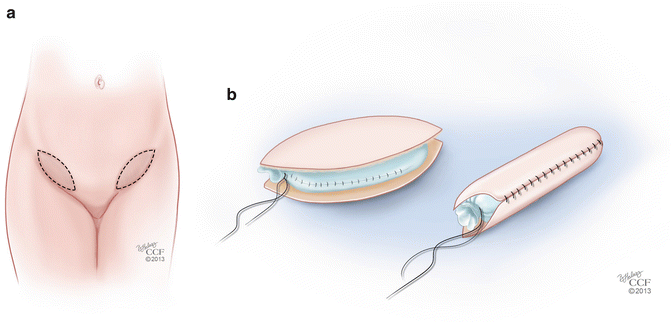

Fig. 18.3
(a, b) Modification of the McIndoe procedure. (a) Technique using full-thickness skin grafts (FTSG) from the inner groin. (b) FTSG placed over a mold (All: Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2013. All Rights Reserved)
The ideal lining for the vagina is a moist mucosa; however, there are limited donor sites for this type of graft. An option includes lining the neovaginal cavity with multiple full-thickness buccal mucosal grafts. The advantage of this type of grafting is that the neovagina is lined with mucosa, which is moist and may facilitate pleasurable intercourse. Additionally, the donor site heals well with virtually no morbidity [37]. The use of autologous buccal mucosa to reconstruct the vagina was presented in 2003 in two separate publications. Lin et al. [38] used complete pieces of full-thickness harvested mucosal grafts (approximately 6–7 cm × 2–3 cm) from both cheeks to line the neovagina; each graft was expanded in size by making stab incisions throughout the grafts, which were sutured over a stent and then placed in an inside-out fashion into the dissected vesicorectal space. The stent is removed once the graft takes to the underlying tissue. Ozgenel et al. [30] described a similar technique of harvesting the same mucosal grafts, expanding them with stab incisions, but then dividing them into several smaller pieces to cover a larger area over the stent. Another technique involves harvesting two buccal grafts, expanding them and then mincing the grafts into tiny pieces and then spreading the micromucosa graft onto the surfaces of gelatin sponge strips which are then placed over a stent and introduced into the dissected cavity, left in place until the graft takes to the underlying tissue [37]. Biologic grafts may also be used to line the neovagina and obviate the need for autologous tissue, which confers many advantages in terms of donor-site morbidity. Acellular dermal allografts such as Alloderm® (LifeCell Corps., Woodlands, TX), porcine dermal grafts such as Permacol® (Covidien, Mansfield, MA), and porcine intestinal submucosa grafts such as Surgisis® (Cook Medical Inc., Bloomington, IN) may be options in neovagina reconstruction. All these grafts are composed of an acellular collagen scaffold that provides a bridge for tissue incorporation and neovascularization. Research on the role of these materials for reconstruction is sparse, with the exception of Alloderm®, which has yielded successful outcomes in vulvovaginal reconstructive cases [39].
Tissue engineering to generate vaginal cells is being studied as an alternative approach to lining the neovagina at the time of the McIndoe procedure. Construction of a functional vagina using autologous cells expanded from a small vaginal biopsy was successful in a rabbit model [40]. And, in 2007, Panici et al. [41] reported the first case of neovaginal construction using autologous in-vitro cultured vaginal tissue. A small skin biopsy can be used to culture vaginal tissue, which can be used as a graft at the time of McIndoe vaginoplasty. Early results demonstrate a vagina with normal length and depth with vaginal tissue present on biopsy [41]. Further research is needed in tissue engineering, in its use for vaginoplasty, and long-term outcomes associated with this type of procedure.
Williams Procedure
The Williams Procedure is a vaginoplasty technique that has been employed in vaginal agenesis patients that involves creating a vulvar skin flap that is then sutured in place to create a neovagina. Williams [42] describes placing allis clamps on the vulvar tissue and applying gentle traction. A U-shaped incision is made extending across the perineum and up to the medial side of the labia (Fig. 18.4a). The upper edge of the incision is made 4 cm laterally and up to the level of the external urethral meatus. The skin flap is sharply dissected off of the underlying tissues creating a flap that can be mobilized inwards, creating a vaginal pouch (Fig. 18.4b). Once the tissues are mobilized, a first layer of sutures is placed between the inner skin margins using interrupted 0 delayed absorbable sutures, starting posteriorly and moving anteriorly (Fig. 18.4c). A second layer of sutures of the same material is used to approximate the subcutaneous fat and the perineal muscles (Fig. 18.4d). Lastly, the external skin is sutured with interrupted stitches (Fig. 18.4e). The Creatsas modification of the Williams vaginoplasty involves using electrocautery to open the hymen at the 3, 6, and 9 O’clock positions, which further opens the introitus and helps to create adequate vaginal caliber and prevents hemorrhage due to rupture of hymenal vessels during the first sexual intercourse [43]. Follow-up of these patients reveals overall subjective satisfaction with vaginal lengths of 10–12 cm and widths of 4–5 cm [44]. This procedure is considered to be superior to the McIndoe procedure as it is can be performed in less time and there is less need for postoperative vaginal dilators, which reduces the psychological impact of the treatment [43].
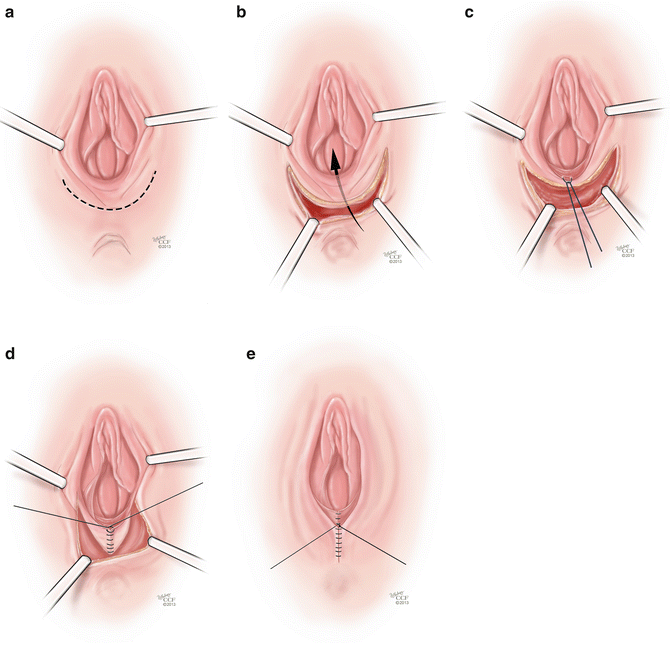

Fig. 18.4
(a–e) Williams procedure. (a) The initial step of the Williams procedure is a U-shaped incision extending across the perineum and up to the medial side of the labia. (b) The skin flap is sharply dissected off of the underlying tissues creating a flap that can be mobilized inwards, creating a vaginal pouch. (c) Once the tissues are mobilized, a first layer of suture is placed between the inner skin margins using interrupted 0 delayed absorbable sutures. (d) A second layer of sutures of the same material is used to approximate the subcutaneous fat and the perineal muscles. (e) The external skin is sutured with interrupted stitches (All: Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2013. All Rights Reserved)
Laparoscopic Procedures
The Vecchietti and Davydov techniques for vaginal reconstruction were first performed as open procedures, but advances in minimally invasive surgery have allowed these procedures to be performed laparoscopically. The main advantage of a laparoscopic approach is the ability to bridge the need to perform an indicated abdominal procedure with the ease of recovery for the patient. The postoperative phase of these surgeries is usually very involved, and therefore, length of hospital stay is usually not significantly shortened, as is seen with most laparoscopic operations. However, once discharged from the hospital, patient recovery is easier as postoperative pain is less and patients are able to return to their daily activities faster.
Vecchietti Procedure
The Vecchietti procedure involves gradual mechanical stretching of the patient’s vaginal skin to create a full-length vagina. This procedure is most appropriate for patients presenting with vaginal agenesis and no prior reconstructive surgery [45]. In this procedure [46], the vesicorectal space is carefully dissected laparoscopically to reflect the bladder anteriorly. An EEA sizer can be placed inside the rectum for mobilization and visualization of the rectovesical space in order to avoid entry into the rectum at the time of dissection. A 2 cm olive-like acrylic bead is placed on the vaginal dimple and is sutured in place to the perineum. Under direct visualization with the laparoscope, a guide needle is used to pass permanent sutures through the acrylic bead and vagina and into the dissected rectovesical space in the pelvis. A guide needle is then inserted suprapubically and used to pull the sutures out of the body (Fig. 18.5a). The sutures are connected to a traction device that is secured to the patient’s abdomen (Fig. 18.5b). Sutures are tightened on a regular schedule, placing traction on the vaginal epithelium, and gradually increasing the length of the vaginal canal. Once adequate vaginal length is achieved, the traction device is removed and the sutures are cut and freed from the body. Patients are advised to practice daily dilation in order to help stretch the vaginal epithelium and maintain vaginal caliber and length during the traction phase of the procedure, and for a limited amount of time once the device is removed. As with the Frank dilation method, patient compliance with vaginal dilation and routine follow-up of the traction device are paramount to the success of the surgery. Data on long-term anatomic outcomes as well as sexual health and quality of life outcomes are favorable with this procedure [47].
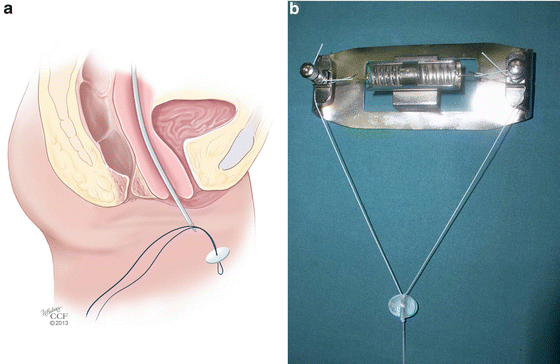

Fig. 18.5
(a, b) Vecchietti procedure. (a) Under direct laparoscopic visualization, a guide needle is used to pass permanent sutures through the acrylic bead and vagina and into the dissected rectovesical space in the pelvis. A guide needle is then inserted suprapubically and used to pull the sutures out of the body. (b) Sutures are connected to a traction device that is secured to the patient’s abdomen (a: Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2013. All Rights Reserved; b: Used with permission from Fedele L, Bianchi S, Berlanda N, Fontana E, Bulfoni A, Borruto F. Laparoscopic creation of a neovagina with the laparoscopic Vecchietti operation: comparison of two instrument sets. Fertility and Sterility 2006; 86(2):429-432)
Davydov Procedure
The Davydov procedure [48] is a technique used to create a neovagina using the patient’s own peritoneum. Good candidates for this procedure include patients with disorders of sex differentiation, such as XY females, who have undergone prior feminizing genitoplasty procedures, but have had poor outcomes, or are not satisfied with vaginal length or caliber. Several modifications of the procedure exist. We recommend first making a U-shaped perineal incision to serve as a landmark for where the peritoneal edges are to be sutured later in the case. Laparoscopic dissection is then done in the rectovesical space, similar to the technique described for the Vecchietti procedure. This is also done using an EEA sizer in the rectum, to delineate the correct dissection plane. Releasing peritoneal incisions in the pouch of Douglas are then made either laparoscopically or transvaginally, freeing and mobilizing the peritoneum caudally so that it can be sutured to the previously made perineal incision. Closure of the abdominal end of the neovagina is done laparoscopically with a purse string suture (Fig. 18.6). A vaginal mold is left in place for several weeks and, once removed, it is replaced with daily dilation until maximal vaginal length is created [45].
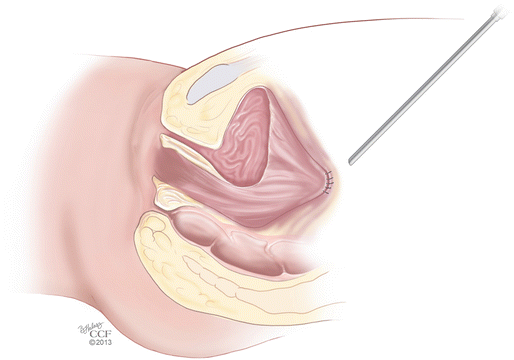

Fig. 18.6
Davydov procedure. The mobilized peritoneum is sutured to the perineal incision and closure of the abdominal end of the neovagina is done laparoscopically with a purse string suture (Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2013. All Rights Reserved)
There are significant advantages to these two laparoscopic procedures when compared to nonsurgical dilation methods. Lengthening of the vagina is accomplished at the time of the procedure, and does not require long-term dilation that can be very uncomfortable initially and time-consuming. The dilation that is then required postoperatively is usually much easier as the vagina has already been created and the main goal of dilation is maintenance of length and caliber. Patients tend to be very compliant with these steps. The main disadvantage is that both techniques require surgical intervention, and while they are performed in a minimally invasive fashion, require extensive dissection into the rectovesical space, which can be associated with rectal, bladder, nerve, and vascular injury. Therefore, meticulous technique is required by an experienced surgeon.
Myo- and Fascio-Cutaneous Flap Procedures
The principle of a myo- and fasciocutaneous flap is the creation of an island flap that depends on the underlying muscle or fascia for its vascular supply. The flap is made up of muscle with or without fascia or fascia alone and the overlying subcutaneous and cutaneous tissues.
Two main techniques can be described when reconstructive surgery is performed using flaps: (1) the standard local or regional flap technique which is based on a vascular pedicle that remains intact while the flap is being mobilized and (2) the more sophisticated microvascular free flap, which involves ligation of the vascular pedicle and reanastomosis to the vasculature of the recipient site. With a few exceptions, the pedicled flap is the most commonly employed flap technique for reconstruction of the neovagina and is usually used after extirpative pelvic surgery or when initial skin-graft techniques have failed in patients with congenital anomalies.
Flap orientation and dimensions of the skin paddle harvested are designed to achieve adequate perfusion through the muscular portion of the flap while achieving adequate skin for vaginal reconstruction, as well as primary donor-site closure. All pedicled flaps have their limitations in terms of the arc of rotation, the size, the tissue volume, and the restriction of mobility. These factors sometimes make it difficult to tailor the flap to the defect that needs repair [49]. Advantages of myo- and fasciocutaneous flaps include the mobilization of a substantial amount of tissue to repair pelvic dead space while providing a source for revascularization for the surrounding tissues. The disadvantages of these flaps are that they can sometimes be very bulky which can affect cosmetic outcome and make the neovaginal cavity narrow, the skin paddles that line the vagina do not provide any lubrication for intercourse, and there can be significant morbidity from the donor site [50].
Rectus Abdominis Flap
Rectus abdominis musculocutaneous flaps are based off of the deep inferior epigastric vessels and can be harvested in two different orientations: transverse (TRAM) and vertical (VRAM). The VRAM flap is usually the preferred method of harvesting for exenteration procedures when stomas are created for bowel and urologic reconstruction [49, 51]. The VRAM flap can be taken from either the patient’s left or right side and is developed above the level of the arcuate line (Soper) (Fig. 18.7a). The skin paddles typically measure 12 × 8 cm in size, which is usually sufficient for the creation of a functional vagina [52]. The horizontal dimension is usually limited by the ability to close the skin primarily; a flap width extending 2 cm lateral to the palpable edges of the rectus can be closed easily in most cases [53]. Once the course of the deep inferior epigastric vessels is identified with Doppler, the flap is elevated from the costal margin to the level of the inguinal fold. Dissection is carried down to the rectus sheath and the lateral border is opened sharply. The skin island with the underlying subcutaneous tissue is mobilized off of the anterior rectus fascia, and the fascia is incised in a slightly smaller ellipse mirroring the skin island in order to leave a smaller fascial defect [51]. The muscle is elevated off of the posterior fascia after the intercostal neurovascular bundles are ligated. The flap is freed superiorly by dividing the muscle at the costal margin. The superior epigastric vessels are identified and ligated. The deep inferior epigastric vessels remain as the vascular supply to the flap, and are identified inferiorly on the posterolateral surface of the muscle, crossing the lateral border of the muscle at approximately the level of the arcuate line. The flap is then elevated carefully so as to not shear the underlying branches of the pedicle. Interrupted absorbable sutures can be used to secure the muscle edges to the overlying subcutaneous tissue to prevent shearing during flap transfer [53] (Fig. 18.7b, c). TRAM flaps are raised in an elliptical fashion as well, below the umbilicus, from one anterior superior iliac spine to the contralateral iliac spine (Fig. 18.8a). In a similar fashion, the dissection is made underneath the anterior rectus fascia, which is preserved in a transverse orientation. The epigastric vessels are identified, and the superior vessels are ligated to allow for mobilization of the pedicled flap in a similar method used for the VRAM (Fig. 18.8b, c).
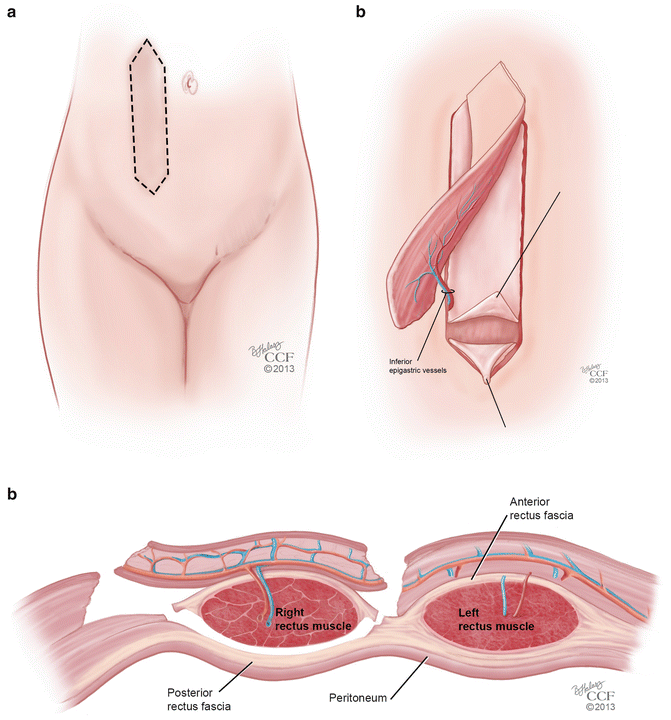
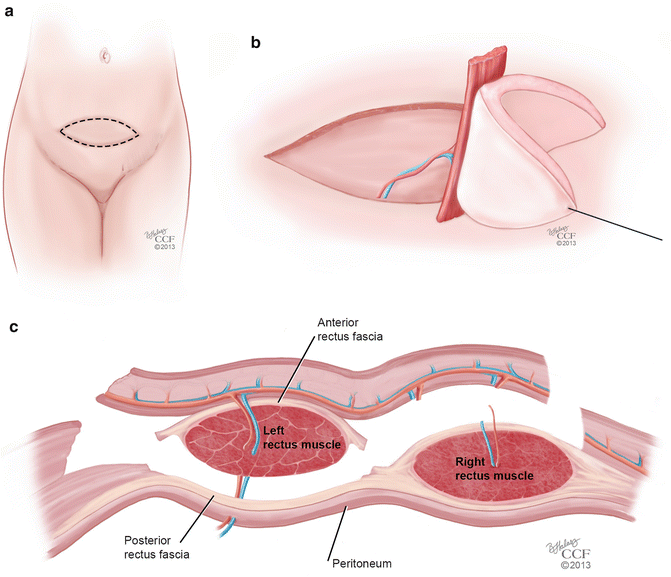

Fig. 18.7
(a–c) VRAM flap. (a) Orientation of the vertical rectus abdominis musculocutaneous (VRAM) flap. (b) Mobilization of the VRAM flap on its vascular pedicle. (c) Coronal view of the mobilization of the VRAM flap on its vascular pedicle (All: Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2013. All Rights Reserved)

Fig. 18.8
(a–c) TRAM flap. (a) Orientation of the transverse rectus abdominis musculocutaneous (TRAM) flap. (b) Mobilization of the TRAM flap on its vascular pedicle. (c) Coronal view of the mobilization of the TRAM flap on its vascular pedicle (All: Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2013. All Rights Reserved)
Once the flap is raised, it is folded into a tube by approximating the edges in a 2-layer closure using absorbable sutures. VRAM flaps are folded into a tube such that the proximal and distal ends of the flap form the introitus once placed in proper position [52]. TRAM flaps are folded such that the lateral border is approximated to the medial border and the cranial edge of the flap is used to form the introitus [51]. The tube is then mobilized into the pelvis through an opening in the posterior rectus fascia, and then brought beneath the pubic ramus, without placing tension on the pedicle. Closed suction drains are placed in the abdomen and pelvis to prevent hematoma and seroma formation. The rectus fascia at the donor site is closed with heavy running sutures and the overlying skin is closed in a manner that limits the distortion of the umbilicus.
A modification to the VRAM flap is the inferior-based VRAM flap. This flap has been shown to meet reconstructive needs in cases of vulvar and perineal defects after resective surgery. Traditional myocutaneous flaps used for reconstruction following radical vulvectomy can cover the large perineal defects but do not provide a “functional” reconstruction that can preserve anal and vaginal patency. The inferior-based VRAM flap is marked and raised in a similar fashion to the standard VRAM flap. The muscle is then split distally in the midline with care taken to avoid transection of the muscular branches of the superior epigastric artery that anastomose with the deep inferior epigastric artery, and supply important perforators to the muscle and skin of the flap. These muscular branches can easily be identified and separated on the underside of the muscle by using spreading dissection, each supplying a tongue of overlying skin and subcutaneous tissue [53]. Division of the distal flap produces well-vascularized myocutaneous fork flaps that can be draped around the vaginal cuff and crossed inferiorly over the perineal body to create a fourchette. This also provides a skin edge for attachment of anal mucosa if extensive perianal dissection was performed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


