(1)
Department of Dermatology, University of Montreal, 3175 Chemin Cote Ste Catherine Montreal, Quebec, H3T 1C5, Canada
Abstract
Tinea capitis is a common infection of the scalp and scalp hairs that produces a variety of symptoms including hyperkeratosis and alopecia. Causative agents cary worldwide but Trichophyton tonsurans has emerged as a significant cause. Therapies include fomite reduction and oral antifungals.
Keywords
Tinea capitisScalp ringwormPaediatric dermatophytosis Trichophyton (T.) Microsporum (M.)TerbinafineGriseofulvinItraconazoleFluconazoleAsymptomatic carriersBackground/Introduction
Tinea capitis is due to an infection of the hairs of the scalp caused by superficial fungi (dermatophytes) of the genera Microsporum or Trichophyton. Synonyms include scalp ringworm and ‘Tinea tonsurans’ [1]. Tinea capitis is characterised by hair loss, with accompanying scaling, hyperkeratosis, and at times inflammatory patches, pustules, and plaques. World incidence has reached epidemic rates and now varies from 4 to 15 % [1].
Epidemiology/Demographics
•
Tinea capitis is the most common paediatric worldwide superficial mycosis with epidemics ranging from 4 to 15 % of the population and pathogenic organisms depend on geographical region.
•
Anthropophilic tinea infections spread between humans of whom asymptomatic carriers and fomites may harbour fungus, and these are increasing with urbanisation and immigration.
•
Zoophilic dermatophyte infections are more common in rural settings, where pets, rodents, and livestock may infect humans.
Tinea capitis is the most common paediatric dermatophyte infection throughout the world [2] World incidence has reached epidemic rates and now varies from 4 to 15 %; frequency of Trichophyton (T.) schoenleinii has been decreasing [1, 3, 4]. There is a slight male predominance [1]. Tinea capitis affects primarily prepubertal children with a peak incidence of 3–10 years, and less commonly adults [1]. Postpubertal children and adults are at least partially protected by the increased sebum with fatty acids which are fungistatic [1, 2]. The fluctuations in the causative agent of tinea capitis may be due to the environment, human migratory patterns and immigration, new therapies, and pathogen and host factors [2, 5–7].
The dermatophytes involved may be acquired by human contact (anthropophilic) or contact with an infected animal (zoophilic). There has been a significant worldwide rise in the incidence of infections caused by anthropophilic dermatophytes and a relative decrease of the zoophilic ones in childhood tinea capitis, as the population becomes more urbanised [7]. The anthropophilic organisms include Microsporum (M.) audouinii, T. tonsurans, T. soudanense, T. violaceum, and the anthropophilic variant of T. mentagrophytes [2, 7].
Whereas cats and dogs harbour M. canis, T. verrucosum is found in cattle, and zoophilic strains of T. mentagrophytes (now called T. interdigitale) are transmitted by a variety of rodents including mice, guinea pigs, hedgehogs, rabbits, and chinchilla. Of note, these T. mentagrophytes infections have been dubbed ‘cuddly toy mycosis’ [8]. The subspecies include var. granulosum (rodents), var. erinacei (hedgehog), and var. quinckeanum (mice) as well as Trichophyton species of Arthroderma (A.) benhamiae found in small rodents, most commonly in guinea pigs [9–11]. Zoophilic T. mentagrophytes have been a cause of neonatal kerion in two Caucasian infants in Argentina [12].
Epidemiologic studies of tinea capitis have been performed worldwide and indicate changing trends and an evolution in the types of species most frequently involved [2] (see Table 18.1). In the United States, Canada, Mexico, Jamaica, Central America, Ireland, and Brazil, T. tonsurans has replaced M. audouinii and M. canis as the most common cause of tinea capitis since the 1960s [2, 13–17]. In the United States, a study of children in Northern California 1998–2007 demonstrated that the annual incidence decreased according to prescriptions, and that the majority of affected patients were African-American with the overwhelming dermatophyte T. tonsurans (91.8 %), followed by M. canis at 3 % [1].
Table 18.1
Geographic location and ethnicity defines the predominant organism responsible for tinea capitis
Country | Dermatophyte predominant |
|---|---|
The Americas (especially African Americans) | T. tonsurans |
Australia, New Zealand | T. tonsurans |
The United Kingdom | T. tonsurans |
Europe | M. canis |
West Africa | T. soudanense |
East and North Africa | T. verrucosum |
Asia (China), Middle East, India | T. verrucosum, M. canis, T. schoenleinii |
In contrast, M. canis is the most frequent pathogen in Europe [3, 18–20]. M. canis has remained most prevalent around the Mediterranean and specifically bordering countries like Belgium [3], Austria, Hungary, Germany, Poland, and Israel [6] as well as Saudi Arabia and Puerto Rico [2]. Anthropophilic tinea capitis is increasing mainly in urban areas in Europe, with T. tonsurans prevalent in the UK [21, 22] and Ireland [15] and increasing in Poland [23] and in Spain [24] second only to M. canis, and T. soudanense and M. audouinii in France [6]. In the UK from 1980 to 2005, frequencies of isolations of zoophilic fungi from scalp infections decreased by 90 % (M. canis, T. verrucosum, T. mentagrophytes var. mentagrophytes) while anthropophilic T. tonsurans and T. violaceum increased by 1,000 % over the same period [21]. T. violaceum is taking hold probably due to immigration from Africa and India, with reports common in Romania [25], Italy [26], Sweden [27], Portugal, Spain [28], Russia, Greece [3, 29], and Yugoslavia [6, 28]. In Sarajevo and Bosnia, over 10 years up until 2006, tinea capitis was due to zoophilic dermatophytes (91.8 %); primarily M. canis prevailed over anthropophilic (7.2 %) T. schoenleinii (2.4 %) and T. violaceum and geophilic (1.0 %) dermatophytes [30]. In Kuwait, M. canis also predominates with 62.5 % followed by T. violaceum with 19.3 % and T. tonsurans with 13.1 %, with T. rubrum being the least frequent [31].
T. violaceum and M. canis are prevalent agents in Asia, India, and the Middle East [2, 5, 7, 28]. In a 16-year (1993–2008) review in southeast China, M. canis (62.4 %) was predominant, followed by T. violaceum (19.0 %), and T. tonsurans (9.8 %) with notable absence of T. schoenleinii [32]. However, in a rural area of western China, five species were identified in school-aged children (in order of frequency: T. violaceum, T. schoenleinii, M. ferrugineum, zoophilic strains of Arthroderma vanbreuseghemii, and T. tonsurans) [33]. T. violaceum has been reported to be the most frequent cause of tinea capitis in India [34].
T. schoenleinii which causes favus was once quite common; however the worldwide incidence has decreased except in some regions of China, Nigeria, and Iran [4]. In Iran up until 2001, T. violaceum was the most common aetiological agent (37.3 %) followed by T. schoenleinii (21.5 %), M. canis (18.6 %), T. verrucosum (14.8 %), T. tonsurans (5.3 %), T. rubrum (1 %), M. gypseum (1 %), and T. mentagrophytes (0.5 %). Increased incidence was correlated with poor socioeconomic position and large family size [7].
In Africa, predominant dermatophytes causing Tinea capitis in children are: T. violaceum from north and east Africa including Ethiopia and Egypt [35], T. soudanense from West Africa, and M. canis are commonly isolated [2, 36]. T. verrucosum is found especially in males from rural areas in Morocco [37]. In a study from the Ivory Coast conducted in school-aged children during a 10-month period, 2007–2008, 2,458 of 17,745 (overall prevalence of 13.9 %) had positive cultures with the most prevalent aetiologic agents T. soudanense, M. langeronii, and T. mentagrophytes (56.7 %, 21.4 %, and 19.7 %, respectively) and occasional T. violaceum (1.4 %) and T. rubrum (0.8 %) [38].
Although T. tonsurans remains the primary dermatophyte causing tinea capitis in the United States, reports of T. violaceum and T. soudanense have been sporadic. Twenty-four cases of T. violaceum and T. soudanense were reported in Baltimore, MD, USA, between 2003 and 2006, traced primarily to immigrants from West Africa [39]. A retrospective review of 189 cases in 2001–2006 in Columbus, OH, showed that tinea capitis was primarily in African Americans and T. tonsurans (88.9 %) was the main causative agent followed by T. violaceum (4.2 %) [36]. In a 1949 series from Boston, MA, T. violaceum was reported to have caused a single case of tinea capitis among 78 cases of fungal scalp infection [28]. Among subsequent reports in the 1950s and 1960s were three outbreaks of tinea capitis due to T. violaceum occurring in a state school in New York [40] and among family members in Detroit, MI [3] and in Texas [29]. Another report from Detroit investigators in the late 1960s described two adult patients with tinea corporis caused by T. violaceum [41]. In Australia, T. tonsurans caught up with M. canis by 1993, and later reports of T. violaceum in Ethiopian, Mediterranean immigrants, and aboriginals have surfaced [42, 43].
Published reports of T. soudanense infections in the United States have been even more infrequent. Two sisters adopted from Liberia were reported in 2003 in Cincinnati, OH, USA, with tinea capitis due to T. soudanense [44]. Although more recent reports describing infections in the United States due to either T. violaceum or T. soudanense are rare, similar trends have been observed in other countries. In New Zealand, where M. canis is the common cause of tinea capitis, 60 cases were reported of primarily T. violaceum and occasional T. soudanense, most of whom were East African refugees from Somalia [40]. Studies from Sweden, Finland, and Belgium have also reported isolation of T. violaceum and T. soudanense from children with tinea capitis, most of whom were African (particularly Somali) immigrants [27, 41, 45].
Anthropophilic Dermatophyte Infections
Anthropophilic fungi are generally transmitted from person to person with spread occurring more frequently in conditions of overcrowding, cosleeping, poverty, and poor hygiene [7, 49]. Certain sports have been linked with spread of tinea capitis through judo in Japan [50] and France [51], and wrestling in Turkey [52]. In addition, fomites such as combs, brushes, and hair-trimming tools may bear the infected spores [53].
Schools, nurseries, and daycares have reported endemics of tinea capitis; in an outbreak in a Swiss school where three cases of tinea capitis were identified to be due to M. audouinii, three family members and five other classmates were found to be asymptomatic carriers of M. audouinii and were treated to prevent further spread [54]. All 27 children with tinea capitis in a primary school in Madagascar due to M. langeronii had contact with an infected schoolmate and 70 % reported to have infected brothers and sisters at home [55]. Tinea capitis is endemic among schoolchildren from Gabon where they have reported 20–26 % incidence, primarily due to anthropophilic species: T. soudanense (29.4 %) was the most prominent species, followed by T. tonsurans (27.9 %) and M. audouinii (25.0 %) [56]. In a T. tonsurans outbreak in a nursery in England, 12 children developed tinea capitis and 7 staff members tinea faciale or corporis [55].
Asymptomatic Carriers
Asymptomatic individuals with high spore load of M. audouinii [54], T. violaceum, or T. tonsurans may be important vectors in the transmission of scalp ringworm because they have the potential to shed large numbers of spores over long periods of time and may be risk factors at both home and school [57]. The prevalence of asymptomatic carriage differs between geographic regions with a rate of 0.1–49 % [57]. In London, England, more than 200 household contacts of children with T. tonsurans tinea capitis were screened; 7.2 % had clinically evident disease, yet 44.5 % had silent fungal carriage on the scalp, most commonly in children less than 16 years of age [22]. One study in the United States demonstrated an overall carrier incidence of 4 %, with the highest incidence of 12.7 % among girls of African-American descent [58]. Another from Milwaukee, WI, with T. tonsurans tinea capitis reported asymptomatic carriage by household contacts of a child with tinea capitis at 16 %, with 41 % of carriers persisting up to 2 months; 32 % of families had at least one member who was a carrier [49].
Clinical Presentation
•
Hair loss (alopecia)
•
Scalp hyperkeratosis
•
Inflammatory
If both 1 and 2 are present, odds ratios 7.5 for tinea capitis
The clinical pattern of presentation of paediatric tinea capitis depends on the type of infection of the hair shaft as well as the immune response of the host (see Table 18.2). It is often asymptomatic but may be pruritic and even painful if a kerion is present. Involvement is usually patchy; however, it can involve the entire scalp. An endothrix infection by Trichophyton often leads to hair breakage causing a ‘black dot’ pattern with minimal apparent inflammation (see Figs. 18.1 and 18.2) [59]. In contrast, M. audouinii which has an ectothrix invasion will show ‘grey patch’ scaling and alopecia (Fig. 18.3a, b) [59]. Patients vary from a seborrhoeic dermatitis pattern to occasional pustules and a boggy plaque kerion (Fig. 18.4). Favus is described as a honeycomb pattern of yellowish thick cup-shaped ‘scutula’ that coalesce to form plaques on the scalp with underlying alopecia and is caused primarily by T. schoenleinii (90 %) [4]. T. violaceum, T. verrucosum, zoophilic T. mentagrophytes (referred to as ‘var. quinckeanum’), M. canis, and geophilic M. gypseum have also been recovered from favic lesions [4]. Both favus and kerion can lead to permanent alopecia. Posterior cervical, auricular, and occipital adenopathy may be present in tinea capitis but is not as essential a finding as once thought [14, 60]. In as many as one-third of cases, annular lesions of tinea faciale or corporis may be found at a distance, in the individual [15] as well as family members.
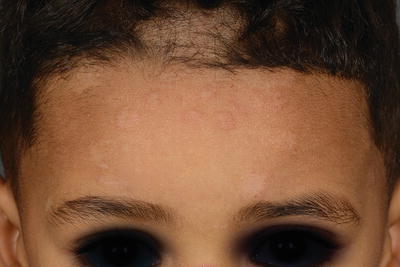
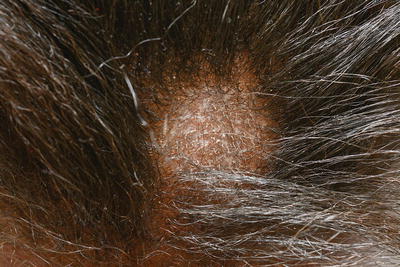
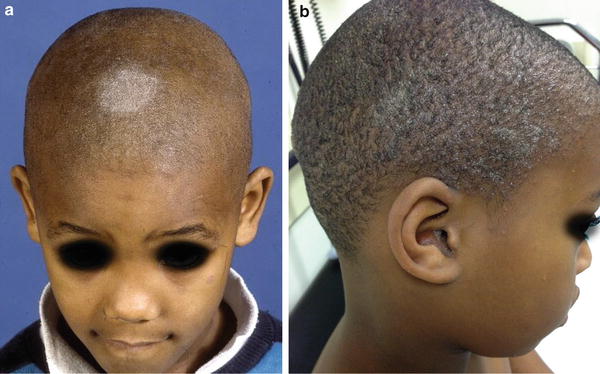
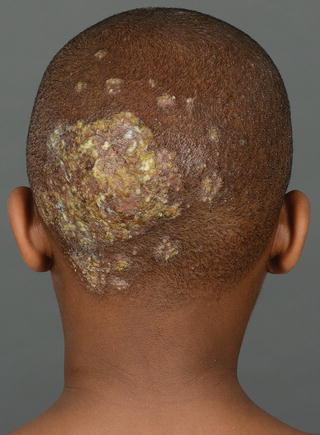
Table 18.2
Clinical presentation of tinea capitis
Non-inflammatory |
Black dot |
Diffuse seborrhoeic dermatitis-like (dandruff-like) |
Grey patches with alopecia |
Inflammatory |
Pustules |
Kerion |
Favus |

Fig. 18.1
Black dot tinea capitis with annular lesions of tinea faciei

Fig. 18.2
Non-inflammatory tinea capitis with localised alopecia

Fig. 18.3
(a, b) Scalp hyperkeratosis (Grey-patch form) of tinea capitis

Fig. 18.4
Kerion and left post cervical adenopathy. Boggy pustular plaque dotted with pustules. Patient admitted for mechanical debridement, compresses, in addition to oral antifungal and antibiotic
According to a 2011 review of 164 children in New York, USA, scalp hyperkeratosis is usually associated with tinea capitis in Black and Hispanic children [14]. This is in contrast to prepubertal Caucasian children, in whom the most common cause of scalp scaling was found to be seborrhoeic dermatitis (54.5 %) followed by atopic dermatitis (24.2 %) and was often accompanied by cervical adenopathy [61]. When scalp hyperkeratosis is accompanied by alopecia in children of colour, the odds ratio for tinea capitis is 7.5 [14]. In a study of 68 primarily African-American children of tinea capitis in an urban setting Norfolk, VA, USA, positive likelihood ratios were 7.5, 3.3, 1.4, and 1.1 for the presence of adenopathy, alopecia, pruritus, and scaling, respectively, for cultures positive for dermatophytes, all T. tonsurans [60].
Acne keloidalis has been described as a novel presentation for tinea capitis in adults [62, 63] and misdiagnoses of impetigo, folliculitis, recurrent furunculosis, pyoderma, folliculitis decalvans, tufted hair folliculitis and dissecting cellulitis has been in the differential diagnosis of some cases of inflammatory tinea capitis [64].
A dermatophytid is a disseminated eczematous eruption which may occur in 5 % of tinea infections before or after initiation of systemic antifungal drug therapy and is not an indication for stopping medication [65]. It is thought to be a delayed type hypersensitivity reaction to the dermatophyte antigen. The eruption may be limited to the face or may be quite widespread, and is composed of monomorphic papules, vesicles, pustules, or pityriasis-like oval patches (Figs. 18.5, 18.6, and 18.7) [65]. Topical and oral corticosteroids and antihistamines may be needed to treat dermatophytids and pruritus; however systemic antifungal therapy must also maintained [65].
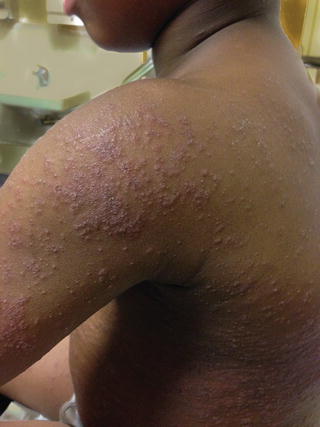
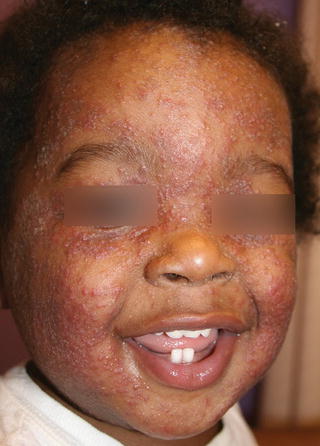
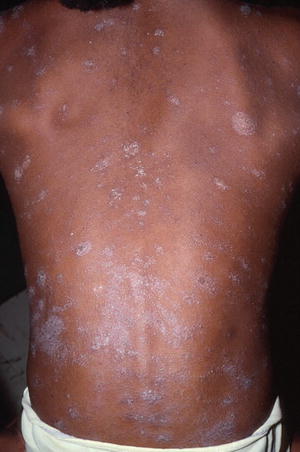

Fig. 18.5
Dermatophytid papulopustular lesions in patient no. 5

Fig. 18.6
Dermatophytid reaction on the face of a child (courtesy of Dr BA Cohen)

Fig. 18.7
Pityriasis rosea-like dermatophytid reaction on the trunk (courtesy of Dr BA Cohen)
Investigations
Complementary investigations can be performed after clinical examination for type of hair loss and/or scale, cervical and occipital adenopathy, and distant tinea faciei or tinea corporis. Wood’s light can be performed to support the diagnosis of tinea capitis. It shows positive fluorescence of the infected hairs in the cases of ectothrix (i.e. M. canis or M. audouinii) (Fig. 18.8) or favus infections, and is absent in the Trichophyton endothrix hair shafts.
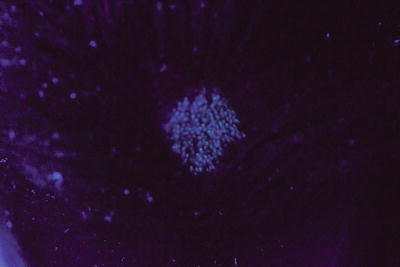

Fig. 18.8
Wood’s light of tinea capitis shows florescence (M. audouinii)
Dermoscopy is a method of increasing importance in the diagnoses of tinea capitis in coloured children in whom erythema is often absent [66, 67]. Corkscrew hairs as a specific dermoscopic sign of tinea capitis in blacks were first described by Hughes (Fig. 18.9a) [68]. In addition, the presence of comma hairs in dark-skinned individuals helps clinch the diagnosis (Fig. 18.9b) [66, 67, 69].
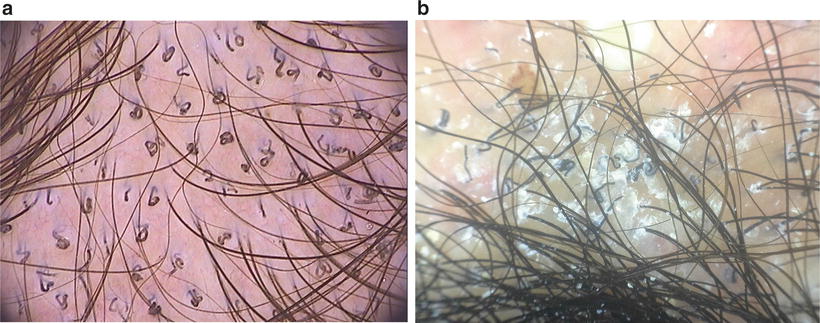

Fig. 18.9
(a) Dermoscopy (trichoscopy) corkscrew hairs (courtesy of Dr A Tosti). (b) Dermoscopy (trichoscopy) shows comma hairs and corkscrew hairs (courtesy of Dr.AM Pinheiro) 2× magnification
Direct microscopic examination of scrapings of hair and scales mounted in 10–20 % potassium hydroxide solution may reveal fungal hyphae and spores; however it may not be performed due to time constraints (Fig. 18.10).
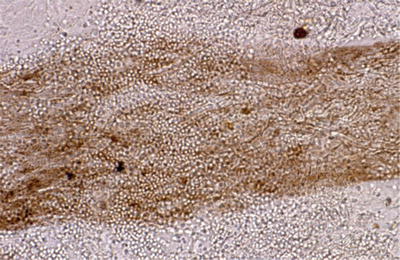

Fig. 18.10
Direct microscopy of ectothrix infection (courtesy of Dr. R Grimalt)
The gold standard to confirm diagnosis of tinea capitis is culture and establishes the subtype, which may have an impact on treatment choice and duration. The method to obtain the culture is varied. Scrapings, hairbrush, toothbrush, and cotton swabs may be used. A retrospective study of 391 children with suspected tinea capitis conducted during 6 years compared the hairbrush method with that of the scalp scraping method; the former was superior, with combination of the two methods increasing the yield of identifying the pathogen [70]. In another study, the hairbrush method had more yield than the toothbrush (P < 0.01) and the cotton swab methods (P < 0.05), and again using both methods provided a higher yield [71]. Although these studies indicate that the hairbrush technique is slightly better, in practice, the cotton swab technique is non-invasive and simple to perform in children, has a high yield, and has been adopted by many clinicians in their office [14, 72].
In a study of 135 children with tinea capitis, the cytobrush was superior to traditional scrapings for detection 97.7 % vs. 85.1 % and speed of growth 8.5 vs. 11.2 days [73]. The cytobrush is a sterile and commercial brush used routinely for cervico-vaginal smears known as the Papanicolaou technique (‘Pap-smear’) and it potentially costs US$0.006 + per unit, even less than sterile blades (US$0.012+), transport swabs (US$0.03+), and toothbrushes (US$0.05+) [74].
Treatment
•
Must always give oral antifungal therapy (4–8 weeks).
•
Griseofulvin has advantages of lower cost, long safety record, and greater efficacy in Microsporum infections; however it may require longer therapy than terbinafine.
•
Complementary therapy for the entire family is important for eradication of spores.
Topical antifungal therapy is inadequate to eradicate tinea capitis because they do not penetrate the hair follicle [75]; however they are complementary to minimise further carriage of spores (see Table 18.3). It has been reported for successful use in a neonate with T. tonsurans tinea capitis [76].
Table 18.3
Treatment of tinea capitis
Oral antifungal |
GRISEOFULVIN some evidence that capsules are more effective than liquid preparation [13] |
Microsized: 20–25 mg/kg/day in bid dosing to a max of 1 g total × 8 weeks |
Ultramicrosized: 10–15 mg/kg/day in bid dosing to a max of 1 g total × 8 weeks
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|