Introduction
The prevalence of thyroid disorders in women of childbearing age and the potential for long-term impact on the offspring exposed to abnormal thyroid hormone levels make it essential that caregivers have a sound approach to identifying and treating abnormal thyroid function during pregnancy. Women may have pre-existing thyroid disease that requires monitoring during pregnancy, have thyroid disease that is not yet diagnosed or treated until after conception, or develop pregnancy-related thyroid dysfunction during or after pregnancy. Thyroid disease may manifest as a change in size of the thyroid gland, a change in level of thyroid hormone or by effects of autoantibodies on the mother or fetus. Interpretation of laboratory test results requires an understanding of the normal physiologic changes in pregnancy in order to prevent mislabeling and avoid inappropriate treatment that may have serious consequences for the developing fetus.
Normal physiologic changes in pregnancy
Pregnancy is associated with significant but reversible changes in maternal thyroid physiology (Table 12.1, Figure 12.1). Thyroid hormone is derived from iodination of tyrosine residues in thyroglobulin to form mono- or di-iodotyrosine which are then coupled to form T4 and T3. The majority of released hormone is T4 and is bound to circulating transport proteins – thyroxine-binding globulin (TBG), thyroxine-binding prealbumin or transthyretin, and albumin. The unbound or free fraction represents 0.04% of total T4 and is the physiologically active hormone or free T4. Thyroid-stimulating hormone (TSH) from the anterior pituitary increases the synthesis and release of thyroid hormone and is the primary regulator of thyroid function.
Table 12.1 Clinical importance of physiologic changes in pregnancy
| Physiologic change | Clinical importance |
| Increased TBG |
|
| Placental de-iodination of T4 |
|
| Increased iodine clearance (renal clearance and fetal transfer) |
|
| Beta-HCG elevation first trimester |
|
| Reduction in TSHRAb during pregnancy |
|
| Postpartum increase in thyroid antibodies |
|
HCG, human chorionic gonadotropin; TBG, thyroid-binding globulin; TSH, thyroid-stimulating hormone; TSHRAb, TSH receptor.
Figure 12.1 Change in thyroid function indices throughout gestation. The shaded area represents the normal range of TBG (thyroid-binding globulin), total T4 (thyroxine), TSH (thyroid-stimulating hormone) and free T4. HCG (human chorionic gonadotropin). Reproduced with permission from Casey [54].
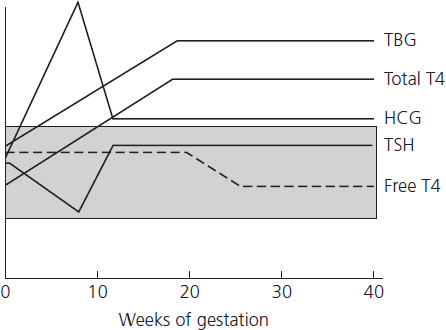
The pregnant woman is required to make 50% more thyroid hormone during pregnancy due to an estrogen-mediated increase in production of TBG [1]. The increased binding of thyroid hormone together with increased metabolism of thyroid hormones by the placenta leads to a greater requirement for thyroid hormone production in order to maintain free T4 levels. Total T4 levels are 150% above the normal nonpregnant reference interval. The increased production of thyroid hormone together with the increased renal clearance and placental transfer of iodine to the fetus in turn results in an increased maternal demand for iodine, an essential building block for thyroid hormone. Women who have borderline iodine deficiency, for example, may be unable to meet this increase in demand, resulting in an overall reduction in thyroid hormone production, a preference for T3 production (the more biologically active form of thyroid hormone, produced from T4 predominantly in extrathryoid tissue), and progression toward hypothyroidism.
Thyroid function studies during pregnancy
Thyroid function tests may be outside the nonpregnant reference range in the normal pregnant woman. Human chorionic gonadotropin (HCG) shares some structural homology with thyrotropin (TSH) and is known to stimulate TSH receptors in thyroid tissue. The rising levels of HCG in the first trimester result in a transient increase in T4 production and subsequent suppression of TSH. It is thus “normal” to have a suppressed TSH and possibly a slightly elevated free T4 during the first trimester. Additionally, there are numerous pregnancy conditions associated with higher than usual HCG levels such as a molar gestation or hyperemesis gravidarum. These conditions may result in an exaggerated stimulation of the thyroid gland and transient first-trimester thyrotoxicosis [2] (see below). Women with twin pregnancies would also be expected to have lower TSH related to the higher HCG in a multiple gestation [3].
The interpretation of thyroid function tests in the second and third trimesters also requires careful consideration because of the known physiologic changes and pitfalls in current assays. TSH will “rebound” to normal nonpregnant levels once HCG returns to a steady state during pregnancy [4]. A majority of studies have demonstrated a subsequent mild decline in free T4 and an increase in TSH after the first trimester and into the third trimester in iodine-replete women. These changes, however, typically remain within the reference range [5]. Importantly, interpretation of the free T4 level and its accuracy is assay dependent and contingent on gestational age and the interference from the progressively increasing TBG level. Free T4 results, and TSH for that matter, should ideally be compared to trimester-specific reference ranges established for specific assays in individual laboratories [6,7]. Some authorities recommend using total T4 levels, rather than free levels, and adjusting the reference interval by 150% compared to the nonpregnant value [8,9]. However, many clinical labs no longer provide total T4 and T3 resin uptake assays (and the associated calculated free thyroxine index (FT4I)) as they are not recommended outside pregnancy.
Fetal thyroid physiology
Embryogenesis of the fetal thyroid gland is largely complete and begins synthesizing thyroid hormone by 12 weeks of gestation. Fetal TSH is also detected at this age and the negative feedback control of thyroid hormone synthesis evolves by approximately mid-gestation. The thyroid–pituitary axis continues to develop throughout gestation with progressive increases in TSH, total T4, and TBG [10].
Especially relevant is the intimate relationship between maternal and fetal thyroid function during the first half of pregnancy. Any requirement for thyroid hormones prior to 12 weeks gestation is supplied by the mother and it is during this time that thyroid hormones are most important to fetal brain development [11]. While overt maternal thyroid failure during the first half of pregnancy has been associated with several pregnancy complications and intellectual impairment in offspring [12–15], it is less clear whether milder forms of thyroid dysfunction have similar effects on pregnancy and infant outcomes [16]. Significant fetal brain development continues considerably beyond the first trimester, making thyroid hormone also important later in gestation. The mother continues to supply thyroxine as well as iodine for fetal thyroid hormone production throughout pregnancy. The role of maternal thyroxine after fetal thyroid production begins is less clear; however, if there is insufficient placental transfer of iodine, fetal hypothyroidism will result.
Because of the association of thyroid antibodies with particular obstetric outcomes and the effect of pregnancy on antibody titers, consideration of the autoimmune nature of most thyroid disease is important in the course of the illness during pregnancy and fetal consequences. There are two main types of thyroid antibodies: those that are directed towards cytoplasmic antigens (thyroid peroxidase (TPOAb) and thyroglobulin (TgAb) antibodies) and those directed to the TSH receptor (TSHRAb). According to the NHANES III data, 10–15% of women of childbearing age, not known to have thyroid dysfunction, will be positive for TPOAB [17]. Another recent study of over 17,000 pregnant women screened in the first half of pregnancy indicates that approximately 5% will have TPO antibodies [18].
Thyroid autoimmunity, with normal thyroid function, has been associated with increased miscarriage rates in some, but not all, studies. This increase in miscarriage does not appear to be related to antibody titer or the presence of thyroid dysfunction. It is hypothesized that the increased risk of miscarriage may be due to:
- subtle maternal thyroid dysfunction
- an underlying autoimmune imbalance reflected by the presence of thyroid antibodies which results in rejection of the fetus
- thyroid antibodies which cross the placenta and directly affect the developing fetal thyroid gland, thus increasing early loss
- increased maternal age of women with thyroid autoimmunity [19].
Pregnancy is associated with a decrease in antibody titers due to trophoblast secretion of immunosuppressant factors [20]. For example, the TSH receptor antibodies associated with Graves’ disease have been shown to decrease substantially during gestation, resulting in clinical improvement and the ability to discontinue medication in many patients. Conversely, antibody titers will increase post partum and are responsible for flares of Graves’ disease in the postpartum period and the development of postpartum thyroiditis (see below) [21,22]. In women who continue to have high titers during pregnancy, passive placental transfer can lead to fetal thyroid disorders after mid-gestation (see below).
Hyperthyroidism complicates approximately 0.2% of pregnancies [23]. Hyperthyroidism is defined by excessive thyroid hormone production due to an overactive thyroid gland. A woman may be tested because of known thyroid disease, the presence of a goiter, symptoms suggestive of thyrotoxicosis or as part of a routine screening strategy. Based on biochemical tests, individuals might be identified with subclinical (suppressed TSH, normal T4 and T3) or overt (suppressed TSH and elevated T4 and/or T3) hyperthyroidism. In women with a depressed TSH yet a normal free T4, hypermetabolic symptoms may rarely be explained by T3 thyrotoxicosis. The most common cause of pre-existing hyperthyroidism is Graves’ disease, an autoimmune hyperthyroidism caused by stimulation of the thyroid gland by thyroid-stimulating hormone receptor antibody (TSHRAb). The differential diagnosis includes toxic solitary thyroid nodule, multiple nodular goiter, exogenous thyroid hormone, amiodarone ingestion, excess iodine intake, and subacute thyroiditis (Box 12.1) [24,25].
Clinical presentation depends on the severity of the thyrotoxicosis and its underlying cause (Box 12.2). Individuals with thyrotoxicosis may present with adrenergic symptoms of stare, lid lag, palpitations, anxiety, nervousness, weight loss, heat intolerance and menstrual irregularity. The thyroid exam may also be helpful when developing a differential diagnosis. For example, a diffuse, symmetric, soft goiter, which may have an audible bruit, is typical of Graves’ disease. A palpable nodule (usually >3 cm) is direct evidence of nodular thyroid disease and generalized thyroid tenderness is consistent with subacute thyroiditis [25]. Associated autoimmune manifestations of hyperthyroidism such as orbitopathy (proptosis, soft tissue periorbital swelling, extraocular muscle dysfunction), pretibial myxedema and clubbing are exclusive to Graves’ disease.
Box 12.1 Causes of thyrotoxicosis in pregnancy
Intrinsic thyroid disease
- Graves’ disease
- Toxic nodule – single or multiple
- Subacute or silent thyroiditis
Excessive, exogenous thyroid hormone
- Factitious
- Therapeutic
Gestational thyrotoxicosis
- Hyperemesis
- Placenta mediated
- Hydatidiform mole
- Multiple gestations
- Hydrops
- Hydatidiform mole
Thyroid storm is an acute, life-threatening exacerbation of thyrotoxicosis. The classic findings are fever, tachycardia, tremor, nausea, vomiting, diarrhea, dehydration, and delirium or coma. Thyroid storm is rare in pregnancy and its diagnosis is based entirely on clinical grounds in women with laboratory tests consistent with overt hyperthyroidism. Heart failure due to cardiomyopathy from excessive thyroxine in women with uncontrolled hyperthyroidism is probably more common in pregnant women [26].
Diagnosis during pregnancy
The clinical features of hyperthyroidism can be easily confused with findings or symptoms typical of pregnancy. Suggestive complaints include nervousness, heat intolerance, palpitations, thyromegaly or goiter, failure to gain weight or weight loss, and exophthalmos. Although nausea is common in early pregnancy, the occurrence of hyperemesis gravidarum together with weight loss may also signify overt hyperthyroidism. Thyroid testing may be beneficial in this circumstance but routine testing in women with hyperemesis gravidarum is not recommended. Importantly, one must consider the impact of gestational age on measurement of TSH. It is important to not overdiagnose hyperthyroidism in the first trimester as 10–20% of women will have a suppressed TSH. Subclinical hyperthyroidism (suppressed TSH with normal free T4) has not been shown to adversely affect pregnancy [27]. At present, there is no convincing evidence that subclinical hyperthyroidism should be treated in nonpregnant individuals and treatment during pregnancy is also unwarranted [28]. Indeed, it should be considered contraindicated because maternal antithyroid drugs cross the placenta and may cause fetal thyroid suppression [29].
Box 12.2 Most common causes of thyrotoxicosis in pregnancy
All causes
- Nervousness
- Agitation
- Fatigue
- Tachycardia/palpitations
- Heat intolerance
- Weight loss
- Increased frequency of bowel habits
- Skin/hair/nail changes
- Skin is soft and moist
- Onycholysis (separation of the distal nail from its bed)
- Hair becomes soft, fine and may thin
- Skin is soft and moist
- Eye signs (distinct from Graves’ ophthalmopathy)
- Lid lag (elicited by having the patient follow your finger as you move it from the top to the bottom of their visual field. Lid lag is present if the upper lid is slow to follow the eye on downward movement and white sclera becomes exposed above the iris)
- Lid retraction and stare (the white sclera above the iris is visible at rest. The orbit appears prominent but is not actually bulging (proptotic))
- Lid lag (elicited by having the patient follow your finger as you move it from the top to the bottom of their visual field. Lid lag is present if the upper lid is slow to follow the eye on downward movement and white sclera becomes exposed above the iris)
Specific to Graves’ disease
- Graves’ orbitopathy: chemosis (swelling of the conjunctiva), proptosis (exophthalmos or bulging orbit), dysconjugate gaze (double vision on looking to the extremes of the visual field)
- Pretibial myxedema (a skin disorder presenting as bilateral, firm, nonpitting, asymmetric plaques or nodules that are most often confined to the pretibial area but may occur anywhere)
- Thyroid bruit
- Clubbing (individuals without clubbing display a diamond-shaped window at the base of the nail beds when two fingers from opposite hands are opposed dorsally. The distal angle between the two opposed nails should be minimal. In individuals with digital clubbing, the diamond window is obliterated and the distal angle between the nails increases with increasing severity of clubbing)
Box 12.3 Indications for measurement of maternal TSH receptor antibodies in pregnancy
First trimester
- Previous radio-active iodine or thyroidectormy for Graves’ disease
- New-onset thyrotoxicosis to differentiate Graves’ disease from gestational thyrotoxicosis
- Previous pregnancy complicated by fetal or neonatal hyperthyroidism
Third trimester
- Woman requiring antithyroid drugs for Graves’ disease into third trimester
Once a diagnosis of biochemical hyperthyroidism is confirmed in pregnancy by a suppressed TSH and an elevated free T4 and/or freeT3, it is important to determine the cause. A woman may have previously undiagnosed Graves’ disease, nodular thyroid disease or a condition unique to pregnancy. Gestational thyrotoxicosis, also referred to as first-trimester thyrotoxicosis or HCG- related thyrotoxicosis, is typically associated with hyperemesis gravidarum. It can also be due to high levels of HCG resulting from molar pregnancy. Both conditions are associated with high HCG levels that lead to TSH receptor stimulation and transient hyperthyroidism. Women with gestational thyrotoxicosis are rarely symptomatic, have minimal thyroid enlargement, and are TSHRAb negative [30]. With expectant management of hyperemesis gravidarum, serum free T4 levels usually normalize in parallel with the decline in HCG concentrations as pregnancy progresses beyond the first trimester. Similar results would be expected with definitive treatment of a molar gestation.
It may be challenging to decide between gestational hyperthyroidism and primary thyroid disease in the first trimester (see Box 12.1). Since radio-active iodine thyroid scanning is prohibited during pregnancy, the clinician must rely more on clinical presentation. If clinical findings are inconclusive, it may be helpful to measure TSHRAb. TSH receptor antibodies are positive in 80% of patients with Graves’ disease [31].
Management
The goal of management of thyrotoxicosis is primarily to normalize, but not suppress thyroid hormone levels and to secondarily treat bothersome adrenergic symptoms of hyperthyroidism. Antithyroid drugs cross the placenta and may cause fetal thyroid suppression, so the minimal dose to achieve adequate metabolic control is preferred. Treatment options for nonpregnant women include treatment for 12–24 months with antithyroid medication, radio-active iodine to partially ablate the thyroid gland, and near-total thyroidectomy. The selection of one of these three depends on patient and clinician preferences, plans for pregnancy as well as clinical characteristics such as severity of thyrotoxicosis, size of the thyroid gland, and presence of ophthalmopathy. For example, in cases of toxic thyroid nodules, radio-active iodine is the preferred choice outside pregnancy. The risk of permanent hypothyroidism is less than when treating Graves’ disease with radio-active iodine, and antithyroid drugs generally do not result in remission of the hyperthyroidism related to thyroid nodules. Moreover, if symptoms related to compression are present with multinodular goiters or there is a question of malignancy in a nodule, a near-total thyroidectomy may be recommended.
Preconceptional counseling
Pregnancy outcomes
Severe thyrotoxicosis is associated with poor obstetric outcomes, but controlled hyperthyroidism is tolerated well during pregnancy [32,33]. Pregnant women with hyperthyroidism are at increased risk for spontaneous pregnancy loss, congestive heart failure, thyroid storm, preterm birth, pre-eclampsia, fetal growth restriction, and associated increased perinatal morbidity and mortality [32,34]. Fetal risks depend on degree of thyrotoxicosis, underlying cause of the thyrotoxicosis and treatment modality used. In most cases, the perinate of a hyperthyroid mother is euthyroid. Placental transfer of TSHRAb, however, can cause fetal Graves’ disease. This manifests as fetal tachycardia, high output cardiac failure, hydrops, craniosynostosis, intrauterine growth restriction (IUGR) and fetal goiter [35,36]. This occurs in approximately 2–10% of offspring of affected women [37]. The risk to offspring is directly related to maternal antibody titer in the third trimester [38]. Fetuses of low-risk mothers, those without detectable serum antibody levels, are rarely identified with goiter and are typically euthyroid at delivery.
Fetal goiter may also result from excessive thio-amide exposure. In this case the fetus is hypothyroid, not hyperthyroid. Current data indicate that thio-amides carry an extremely small risk for causing hypothyroidism in the neonate [39,40] and at least four long-term studies have found no abnormal intellectual and physical development in these children [41].
Chronic exposure to hyperthyroidism from inadequately treated maternal hyperthyroidism may impair maturation of the fetal hypothalamic-pituitary-thyroid axis. This can lead to central congenital hypothyroidism in the infant [42].
Thio-amide drugs
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


