Introduction
Critical care refers to the intensive monitoring and aggressive and often invasive treatment of severely ill patients with potentially fatal but reversible conditions, usually in the intensive care unit (ICU). The critically ill obstetric patient presents several challenges. Common illnesses may present differently in pregnant women due to altered anatomy and physiology [1,2]. The impact of less severe disorders may be more devastating due to decreased physiologic reserve during pregnancy [3,4]. Thresholds for instituting therapy may differ in pregnant women because of the threat to fetal well-being [5]. Finally, some diagnostic modalities and drugs which are commonly used in the ICU may have undesirable effects on the fetus [6].
Epidemiology of critical illness in pregnancy
Acute disorders requiring ICU admission complicate 1–9/1000 pregnancies [7]. ICU admission rates range from 100 to 1700 per 100,000 deliveries in individual series [8,9]. Admission rates are generally higher in ICU with a large referral base [9–11] and in hospitals with dedicated obstetric ICU [12,13]. While intercurrent medical disorders account for some of these critical illnesses, a large proportion of women have obstetric disorders like pre-eclampsia, which can usually be detected before they reach a critical stage. In most economically developed countries, a combination of meticulous antenatal care, excellent obstetric anesthesia, skilled intrapartum management and high-quality neonatal intensive care has decreased maternal mortality rates to <20 per 100,000 deliveries [8,14,15]. The absence of one or more of these critical components of care contributes to the extremely high maternal and perinatal morbidity and mortality in underdeveloped countries [10,16].
Organization of the intensive care unit
Care of critically ill obstetric patients involves a multidisciplinary team consisting of an obstetrician or maternal-fetal medicine specialist familiar with obstetric physiology and its derangements, an intensive care physician trained in the detection and treatment of failing organs, an anesthesiologist for pain control, airway management and for anesthesia during emergency surgical delivery, and a neonatologist to manage a preterm neonate who may have suffered intrauterine insult due to severe maternal illness. Nurses with experience in a labor and delivery unit and additional training in advanced life support techniques are essential [17]. The nurse-to-patient ratio must be at least 1:1; two nurses (ideally one with an extensive labor and delivery background and the other with an extensive intensive care background) will be required to provide optimum care during delivery [17]. Ideally a senior member of both the obstetric and intensive care teams should interface in person at least daily to discuss these cases directly to ensure that both team members have a good understanding of the other’s concerns.
Proximity to the labor suite or obstetric operating room is an issue that needs attention. In a majority of hospitals, obstetric patients are managed in the general ICU [8]. Equipment to monitor uterine activity and fetal well-being is usually transported to the ICU when needed. In the 1990s there was great enthusiasm for having a dedicated ICU for obstetric patients [13]. It is now believed that such units may only be justified in hospitals that receive >100 admissions/year requiring obstetric intensive care; smaller numbers may not allow staff to maintain their critical care skills [17]. Moreover, to be economically viable, at least 1% of obstetric hospital admissions would have to be admitted to the ICU [9,13]. Even with admission rates above 100/year, individual familiarity with the management of many critical care cases will still be lacking and the best practice for obstetric critical care units may need to involve formal arrangements to allow co-management of obstetric patients in the obstetric intensive care by intensivists and obstetricians in collaboration with a bedside team of both an obstetric and a critical care nurse.
Indications for intensive care unit admission
The Society of Critical Care Medicine guidelines assign highest priority for ICU admission to unstable patients who need intensive monitoring or treatment that cannot be provided outside the ICU [18]. These would include women with hypotension, coagulopathy, renal failure, hepatic encephalopathy, coma, seizures or respiratory failure. The next priority is assigned to patients who require intensive monitoring and may potentially need immediate intervention [18]. Women with severe pre-eclampsia, HELLP syndrome, jaundice, severe community-acquired infections and pre-existing medical disorders fall in this category [19]. Conditions commonly leading to organ dysfunction and ICU admission in pregnancy are listed in Table 23.1. Previous studies have shown that maternal age >35 years, high parity, multiple pregnancy, ethnic minority status, and transfer from another hospital for delivery are associated with increased need for ICU admission [8,15,20,21].
Table 23.1 Important conditions that may cause severe organ dysfunction or failure during pregnancy or postpartum period
Adapted from Soubra & Guntupalli [15].
| Obstetric disorders | Increased susceptibility during pregnancy | Pre-existing conditions that may worsen during pregnancy |
| Obstetric hemorrhage | Renal | Cardiovascular |
| Adherent, retained placenta | Acute renal failure | Valvular disease |
| Pregnancy-induced hypertension | Infections | Coarctation of aorta |
| HELLP syndrome | Urinary tract infection | Systemic hypertension |
| Acute fatty liver of pregnancy | Listeriosis | Congenital cyanotic heart disease |
| Chorioamnionitis | Viral hepatitis E | Ischemic heart disease |
| Septic abortion | Plasmodium falciparum malaria | Pulmonary hypertension |
| Puerperal sepsis | Varicella pneumonia | Respiratory |
| Amniotic fluid embolism | Hematologic | Cystic fibrosis |
| Intrauterine fetal demise | Disseminated intravascular coagulation | Lung transplant |
| Ruptured ectopic pregnancy | Postpartum HUS/TTP | Renal |
| Pelvic septic thrombophlebitis | Venous thrombosis | Chronic renal insufficiency |
| Peripartum cardiomyopathy | Endocrine | Endocrine |
| Tocolytic-induced pulmonary edema | Gestational diabetes | Prolactinoma |
| Uterine inversion | Sheehan’s syndrome | Diabetes mellitus |
| Neurologic | Liver | |
| Intracranial hemorrhage | Cirrhosis | |
| Respiratory | Hematologic | |
| Pulmonary thromboembolism | Sickle cell disease | |
| Air embolism | Rheumatologic | |
| Aspiration | Scleroderma | |
| Polymyositis | ||
| Neurologic | ||
| Epilepsy | ||
| Intracranial tumors |
HELLP, hemolysis, elevated liver enzymes, low platelets; HUS, hemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura.
Transfers within hospital from one unit to the critical care unit are potentially dangerous times when errors may be made. Clear hand-over communications between physicians and nurses are essential. Transport of patients should be carried out by experienced medical personnel who have ready access to the following equipment while en route: an adequately full oxygen tank and appropriate oxygen delivery device, a bag and mask should the patient stop breathing, continuous cardiac monitoring, a manual suction device, manual blood pressure cuff and stethoscope, good intravenous access, and any medications that might reasonably needed during the time frame involved in the transfer. This list is summarized in Box 23.1.
Obstetric versus medical disorders
Primary obstetric disorders account for 50–80% of ICU admissions during pregnancy and the puerperium in all parts of the world [8,15]. Over 80% of these are due to pre-eclampsia and its complications, obstetric hemorrhage and pelvic sepsis. On the other hand, nonobstetric or medical disorders in pregnancy show large geographic variations. In developed countries, asthma [16,20], pneumonia [12,16], drug abuse [16,22], complicated urinary infections [12,16], pre-existing autoimmune disorders, chronic pulmonary disease [23], endocrine disorders [12], trauma [24,25] and pulmonary thromboembolism [3,26] are common. Medical disorders commonly seen in developing countries include severe malaria, vital hepatitis, cerebral venous sinus thrombosis, tetanus, tuberculosis, rheumatic valvular heart disease, anemia and attempted suicide (poisoning or burns) [8,10,27].
Box 23.1 Checklist in preparation for transfer of patient to an intensive care setting
- Full oxygen tank
- Device to provide oxygen and a double check to ensure it is working (nasal prongs/mask/“100% nonrebreather”/transport ventilator)
- Bag-mask to ventilate patient should breathing cease in transport
- Oxygen saturation monitor with good waveform and alarms on
- EKG monitor with alarms on and adequate paper to print
- Transport defibrillator with batteries checked
- Adequate volume in any active intravenous drips for a transfer that includes unanticipated delays en route
- Syringes, intravenous fluid, and medications that may be needed in transit (e.g. fluid bolus for sepsis patient, an antihypertensive for a pre-eclamptic patient, a benzodiazepine for a patient with recurrent seizures)
- Critical care team has expressed their readiness to receive patient and have received or will receive detailed hand-over including list of medications and results of recent testing
Final “pause” before mobilizing:
- Patient vitals are stable (if patient recently unstable, consider having physician accompany transfer)
- Patient able to protect airway or airway that has been placed is secure
Intensive care units in developed countries are increasingly seeing a unique subgroup of pregnant women. Advances in healthcare have resulted in survival to child-bearing age of women with disorders such as surgically corrected complex congenital heart disease, organ transplant and chronic disorders like cystic fibrosis. Pregnant women with these conditions develop increased morbidity and sometimes require intensive medical care [28,29].
Antepartum versus postpartum intensive care unit admissions
Postpartum women comprise approximately 60–80% of obstetric ICU admissions [8,10,15]. Almost 80% of postpartum admissions occur during the first 24 hours after delivery, usually for obstetric disorders like postpartum hemorrhage and its consequences, aspiration and eclampsia [8]. Late postpartum admissions are usually for puerperal sepsis, cerebral venous thrombosis, acute renal failure and worsening thrombocytopenia in women with the HELLP syndrome [10,30]. Antepartum admissions are more frequently due to co-existing medical disorders. Some of these disorders may precede the pregnancy (e.g. valvular heart disease, asthma, epilepsy) while others may develop acutely during pregnancy (e.g. peripartum cardiomyopathy, viral hepatitis, severe community-acquired pneumonia, acute pyelonephritis) [15,16]. Respiratory failure is the most common problem seen in antepartum ICU admissions [10–12.22].
Initial assessment of a critically ill patient
As in a nonpregnant critically ill patient, the initial assessment of a parturient is focused on airway, breathing, and circulation.
Airway
Airway evaluation and management remain the first priority. Supplemental oxygen may be required in some patients. Tracheal intubation is needed in the setting of persistent hypoxemia, airway obstruction, impaired laryngeal reflexes or altered mental status [31,32]. Pregnant women are at high risk for aspiration of gastric contents and are often more difficult to intubate than nonpregnant patients. Therefore, endotracheal intubation should be performed sooner rather than later, to protect the airway, and should be attempted by the most experienced individual available (who should be notified early of the potential need for intubation) [32–34]. Box 23.2 provides a checklist to ensure the right equipment is available in the room when intubation is attempted in areas where such care is not routine. If the airway exam indicates that tracheal intubation is likely to be difficult [32], awake intubation should be performed with good topical anesthesia [31,33]. Rapid-sequence induction with cricoid pressure and orotracheal intubation is recommended in the obtunded or unconscious parturient without a potentially difficult airway [31,33]. A set of instruments for difficult airway management must always be available in the ICU and the labor and delivery room [32,34].
Box 23.2 Checklist in preparation for endotracheal intubation
For maternal monitoring
- Continuous EKG monitoring of mother
- Blood pressure monitor on patient’s arm
- Pulse oximeter
For resuscitation
- High-flow oxygen source
- Securely fitting mask with manual inflation bag
- Two large-bore (18 G or greater) intravenous catheters in place
- Plan in place for next step if attempt at endotracheal intubation fails (e.g. laryngeal mask airway)
For person doing intubation
(who should be the most experienced person available and have an assistant working with them)
- Oropharyngeal suction equipment
- Laryngoscope with light working
- Stylet
- Gloves, mask, goggles and gown for person doing intubation
- Medications for sedation and (if necessary) paralysis of patient (e.g. IV thiopental 3–5 mg/kg for sedation, etomidate 0.3 mg/kg over 60 seconds for muscle relaxation and succinyl choline 1–1.5 mg/kg up to 150 mg for paralysis)
For verification of correct tube placement
- Ideally a method of verifying that CO2 is being exhaled from endotracheal tube but chest X-ray and bilateral auscultation may be used as additional assessment tools
Breathing
Adequacy of respiration must be established rapidly. Supplemental oxygen and bag-mask ventilation may be required initially. Noninvasive positive pressure ventilation is an option for some patients (see Chapter 1). If respiratory effort is inadequate, tracheal intubation is performed and mechanical ventilation initiated.
Circulation
Hypotension and shock should be treated promptly in order to maintain uteroplacental perfusion. After 20 weeks of pregnancy, pressure of the gravid uterus on the inferior vena cava and abdominal aorta in the supine position can decrease cardiac output by up to 30% [2,4,35]. The parturient should therefore be positioned on either the left or right side. Two large-bore intravenous cannulae (14 G or 16 G) should be placed to administer fluids and a Foley catheter should be placed to monitor urine output [36]. Central venous access may be needed for volume resuscitation, bolus drug administration and infusion of vasopressors. Femoral vein catheterization should be avoided due to the risk of thromboembolism and sepsis [37]. The jugular route is preferred over the subclavian in patients with coagulopathy as the subclavian site cannot be compressed in the event of excessive bleeding or accidental arterial puncture [37]. Hypotension is treated by aggressive volume resuscitation [3,6,36]. If hemorrhage is life-threatening, blood group O Rh-negative packed red blood cells are transfused until type-specific or cross-matched blood is available [38]. It is preferable to place an arterial line at the earliest opportunity to measure the blood pressure continuously. Severe maternal hypotension may require treatment with vasopressors [39]. In pulseless, severely hypotensive patients, intravenous epinephrine (0.5–1 mg) may be given [40].
Comprehensive maternal and fetal evaluation
After stabilization of the airway, breathing and circulation, a thorough evaluation with a detailed history and physical examination is performed. Routine ICU monitoring includes electrocardiogram (EKG), pulse oximetery and noninvasive blood pressure monitoring. Besides blood grouping and cross-matching, blood should be sent for analysis of arterial pH and blood gases, hemoglobin concentration, electrolytes, glucose, renal and liver function. Platelet count, prothrombin time (PT), partial thromboplastin time (PTT) and serum fibrinogen and fibrin degradation product levels are ordered (DIC screen). Thromboelastography (TEG) is an alternative test that measures viscoelastic properties of clot formation and lysis and can diagnose thrombocytopenia, platelet dysfunction and coagulation factor abnormalities [41]. The Kleihauer-Betke test is done in Rh-negative mothers with trauma to look for fetomaternal transfusion. Ultrasonography is performed to evaluate fetal and uteroplacental status and for any abdominal disease.
Methods of assessing and ensuring fetal well-being are reviewed in Chapter 49. There is no standard practice for how fetal monitoring should be carried out in the setting of critical care for patients. The plan should be individualized based on the condition of the mother, the gestational age of the pregnancy, and the preferences of the patient or her healthcare proxy which will determine the purpose of fetal monitoring. Generally, the primary concern and intervention strategy should be to optimize maternal health as this will generally be the safest and most effective route to improving fetal status. At gestational ages prior to ex utero viability, continuous fetal monitoring is difficult to perform and interpret and would not be used as the basis for emergency delivery. Evidence of apparently nonreassuring fetal assessment might be used only to help optimize maternal physiology (e.g. blood pressure and volume status) for maximal fetal and maternal benefit. Once a gestational age has been reached that is compatible with ex utero survival, frequent or even continuous assessment of fetal well-being may be appropriate to inform a decision for delivery for fetal benefit as well as to optimize maternal physiology. Decisions about moving toward delivery should be based upon both fetal assessment and an assessment of the risk of delivery to the mother. Decisions regarding delivery under these circumstances can be among the most difficult in maternal-fetal medicine and should be made in consultation with a multidisciplinary team ideally including intensivists, anesthesiologists, any specialists appropriate for the maternal condition (e.g. cardiology, neurology, etc.), neonatologists and maternal-fetal medicine. If preterm delivery is anticipated and there is no medical contraindication, betametasone (two intramuscular doses of 12 mg, 24 hours apart) may be given to enhance fetal lung maturity [42].
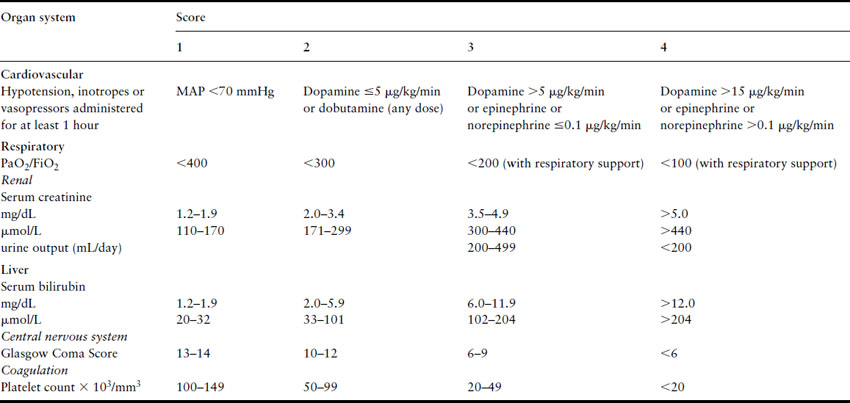
Approximately 65% of obstetric ICU admissions have failure of at least one organ system and 33% have multiple organ failure [8,15]. Organ failure may result from worsening of pre-existing medical diseases like valvular heart disease or onset of a new illness like pneumonia. Many obstetric conditions also may precipitate acute severe organ dysfunction (Table 23.2) [8]. The pattern of initial organ failure in most diseases is predictable. However, it must be realized that function of most organs is interdependent and prolonged failure of one or more organs may secondarily result in a domino effect, culminating in severe multiple organ system failure [43,44]. The Sequential Organ Failure Assessment (SOFA) system is commonly used to define organ failure (Table 23.3) as it permits quantification of individual organ dysfunction as well as a combined assessment [45].
Table 23.2 Pattern of severe organ system dysfunction in obstetric disorders. Although the organs primarily affected in each obstetric disorder are indicated here, prolonged shock and severe DIC may ultimately result in secondary dysfunction of almost all organ systems
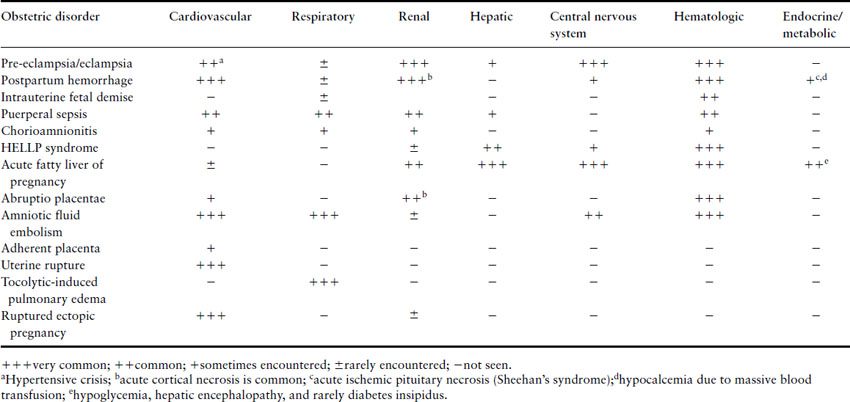
Table 23.3 Sequential Organ Failure Assessment (SOFA) criteria for diagnosis of organ dysfunction or failure. Each of the six organ systems is assigned scores ranging from 0 (normal function) to 4 (severe failure). Scores 1 and 2 indicate organ dysfunction and 3 and 4 indicate organ failure. The scores of all six systems could be added to give a composite score which has prognostic significance
The frequency of failure of individual organs shows considerable geographic variation. In an American ICU, Afessa et al. observed that the most common organ system to fail was the respiratory system (32%) followed by hematologic (28%), cardiovascular (28%), renal (9%), liver (8%), and neurologic failure (1.5%) [22]. In Indian ICU patients, neurologic failure is the most common (63%) followed by hematologic (56%), renal (49%), respiratory (46%), cardiovascular (38%) and hepatic failure (36%) [16]. An Argentinean study found that respiratory failure was the most common (37%) followed by cardiovascular (28%), hematologic (13%) and renal failure (12%) [11].
Shock
Shock is the most common manifestation of cardiovascular dysfunction in obstetric ICU patients (Box 23.3). Shock presents as tachycardia, tachypnea, hypotension, oliguria, altered mental status and lactic acidosis [42,44,46]. Orthostatic hypotension can be the only manifestation of early hemorrhagic shock; the diagnosis may be missed in the supine patient. Signs of external or internal hemorrhage may be present. Rales on auscultation are found in left ventricular failure or acute respiratory distress syndrome (ARDS), a third heart sound in peripartum cardiomyopathy and pulmonary thromboembolism, and cardiac murmurs in valvular heart disease.
Box 23.3 Causes of shock in obstetric patients
| Hypovolemic shock | Hyperemesis gravidarum, ruptured ectopic pregnancy, concealed placental abruption, placenta previa, postpartum hemorrhage, uterine rupture, trauma |
| Septic shock | Chorioamnionitis following intrauterine fetal demise, puerperal sepsis, septic abortion, community-acquired pneumonia, pyelonephritis |
| Cardiogenic shock |



