Introduction
The rheumatologic diseases are a diverse group of ailments that are unified by their pathophysiology. Each is driven by a deregulated immune system that results in autoimmunity. The majority of rheumatologic diseases affect women more often than men, though the cause for this inequity is not clear. Many autoimmune diseases are diagnosed during the reproductive years. As our therapies for these diseases have improved in recent decades, more women with rheumatologic disease desire pregnancy. Fortunately, most pregnancies in women with rheumatologic disease are successful and result in the births of healthy babies to healthy mothers. However, many women will have a worsening of disease activity during pregnancy, prompted by the hormonal and immunologic shifts in pregnancy and compounded by the avoidance of some medications during this period. For optimal care, these women require careful monitoring by both a high-risk obstetrician and a rheumatologist throughout pregnancy.
Epidemiology
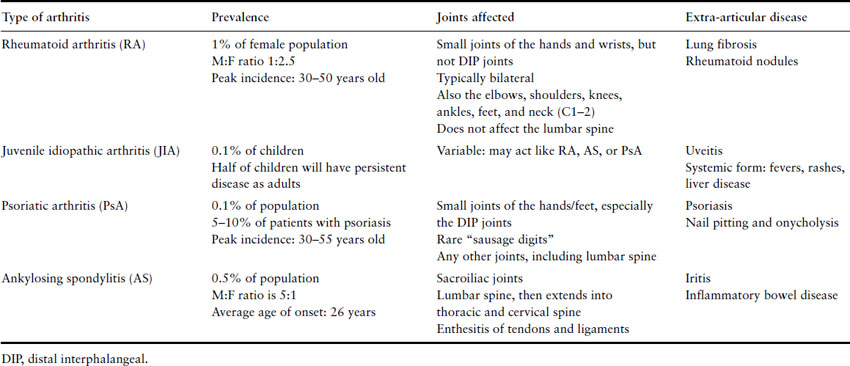
Inflammatory arthritis is a general term that encompasses women with rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), psoriatic arthritis (PsA) and ankylosing spondylitis (AS). The hallmark of each of these diseases is chronic autoreactive inflammation within joints. RA, which affects an estimated 1% of the female population, is the most common form of inflammatory arthritis. The prevalence is 2.5-fold higher in women than in men, and the peak incidence is between the ages of 30 and 50 [1]. JIA first presents in children under age 16, but half of these patients will have persistent joint inflammation as adults. There are an estimated 35,000–50,000 cases of adult JIA in the United States [1]. PsA affects about 0.1% of the US population, and occurs in about 5–10% of all patients with psoriasis. Men and women are affected equally. The peak incidence is between 30 and 55 years of age [1]. AS is fivefold more common in men than women, but may be present in up to 1% of all Caucasians. The prevalence of AS reflects the prevalence of the genetic marker HLA-B27, which is present in the vast majority of patients with this disease [1].
Etiology/pathophysiology
Though the initial trigger for each of these diseases is unknown, once tolerance has been broken, joint inflammation appears to be a self-propagating process. The inflammatory process is accompanied by an influx of inflammatory cytokines, including tumor necrosis factor (TNF)-alpha, interleukin (IL)-1 and IL-6. These cytokines prompt changes in the differentiation of osteoclasts, chondrocytes and collagen. These changes induce the growth of the joint pannus (granulation tissue in the joint), which leads to the swelling that can be seen clinically. It also leads to joint destruction, which can be observed as erosions surrounding a joint on X-ray. Eventually, the destruction results in a distortion of the joint mechanics, loss of range of motion and disability.
Diagnosis
Inflammatory arthritis is diagnosed clinically based on the patient’s history and physical examination. Patients will report significant morning stiffness that lasts usually an hour or more and improves with heat, such as a hot shower, and movement. The pain in the joints can be severe and is accompanied by swelling and warmth. Joints will also have limited range of motion either from pain secondary to inflammation or from alteration in joint structure. The location of the inflammation helps to identify the specific type of arthritis (Table 8.1).
Table 8.1 Inflammatory arthritis: distinctions between the types of arthritis
Laboratory tests can also help to clarify the diagnosis. Patients with inflammatory arthritis will often have an elevated erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP). During pregnancy, the ESR is often elevated regardless of inflammatory disease, so is not useful for monitoring or diagnosing disease. The CRP is not as well studied, but may be more reliable in pregnancy.
The autoantibodies associated with rheumatoid arthritis are the rheumatoid factor (RF) and the cyclic citrulinated peptide (CCP) antibody. The RF is present in 75–80% of RA patients at some point, but can also be positive for reasons other than RA. A positive RF in the absence of significant joint pain and swelling probably does not indicate rheumatoid arthritis (Box 8.1). The CCP antibody is a more recently discovered marker for RA. A little more than half of all patients with RA have the CCP antibody, but only 10% of tests are falsely elevated. Over 95% of patients with both a positive RF and positive CCP antibody will be diagnosed with RA [2].
Inflammatory arthritis in pregnancy
Rheumatoid arthritis is the best studied inflammatory arthritis in pregnancy. Most reports demonstrate an improvement in joint inflammation during pregnancy. A report of 140 women with RA found that by the third trimester, two-thirds had significant improvement in symptoms. Within 1 month post partum, however, two-thirds of women had relapsed and by 6 months, over three-quarters had worsened joint activity [3]. A recent study of 78 pregnancies in women with RA found a more modest improvement in disease activity. Only 11% had major improvement, 37% had moderate improvement, while 45% had either no improvement or worsened [4]. Women with JIA tend to improve, similar to women with RA [5].
Box 8.1 Causes of a positive rheumatoid factor
| Autoimmune disease |
|
| Infections |
|
| Malignancy |
|
Carrying a pregnancy does not worsen the long-term severity of RA. In fact, one study found that women who had a pregnancy during the course of RA had fewer joint abnormalities on X-ray and had better physical functioning than women who had not been pregnant [6]. This study may be biased as women with more severe RA may avoid pregnancy, but the results are reassuring that pregnancy does not worsen the condition.
Women with AS may have increased pain and stiffness during pregnancy. Though the data are scarce for these pregnancies, it appears that women with AS may have more pain than women with RA during pregnancy [7,8]. PsA is also rarely studied in pregnancy. However, small studies show improvement in symptoms in many women [5].
Few fetal complications have been reported in women with inflammatory arthritis, but the rate of preterm birth may be increased in this population. In a nationwide study of RA pregnancies, about one-quarter of pregnancies were born preterm (prior to 37 weeks gestation), compared to 5% of healthy controls [9]. The risk of spontaneous pregnancy loss, however, is not significantly increased in this population. Infant birth weight may be lower among women with RA [10]. Women with active RA who required prednisone during pregnancy had, on average, 400 g smaller babies than healthy women [9,11]. Women with RA do have an increased risk for hypertension and pre-eclampsia in pregnancy, though the risk is more modest than in other rheumatologic diseases [10,12].
Systemic lupus erythematosus (SLE) is an autoimmune disease that primarily affects women in their childbearing years. SLE affects about 0.05% of the population, but the incidence is threefold higher among African American women [13]. The peak incidence is between 15 and 40 years old with a female to male ratio of 9 to 1.
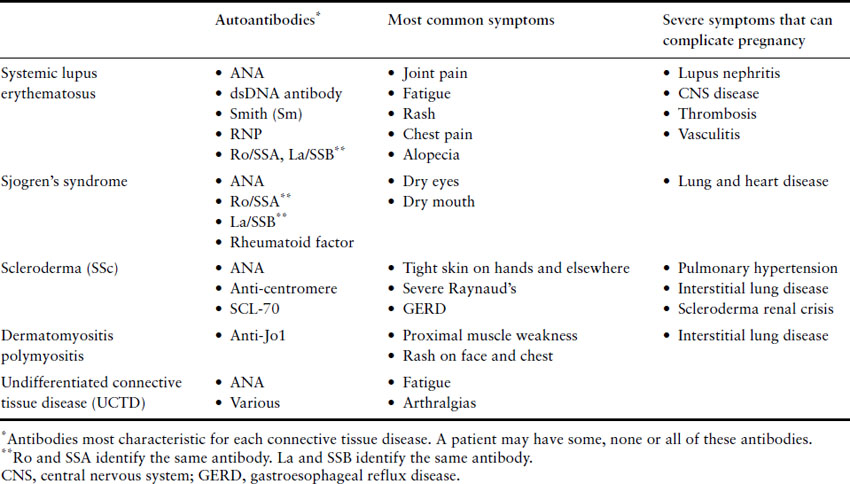
Systemic lupus erythematosus is diagnosed through a combination of signs, symptoms and laboratory findings. According to the criteria developed by the American College of Rheumatology (ACR), a woman must have at least four of 11 criteria to be labeled as lupus (Box 8.2) [14]. Over 95% of women with SLE will have a positive antinuclear antibody (ANA) finding. Other antibodies, including the dsDNA, Ro/SSA, La/SSB, RNP and Smith antibodies, are not required for an SLE diagnosis, but are helpful when present. A woman with some symptoms of SLE, but not enough to fulfill the ACR criteria, may be given the label of undifferentiated connective tissue disease (UCTD). In most cases, UCTD is a milder illness with only a small subset of patients progressing to full SLE in the following years [15]. We recommend consultation with a rheumatologist to establish a lupus diagnosis.
The course of SLE, both during and outside pregnancy, can be unpredictable. Some women will have significant fatigue and arthralgias, without internal organ damage. Others may have a fulminant course with rapid progression to endstage renal disease. Close monitoring by a rheumatologist is very important to evaluate lupus and direct treatment.
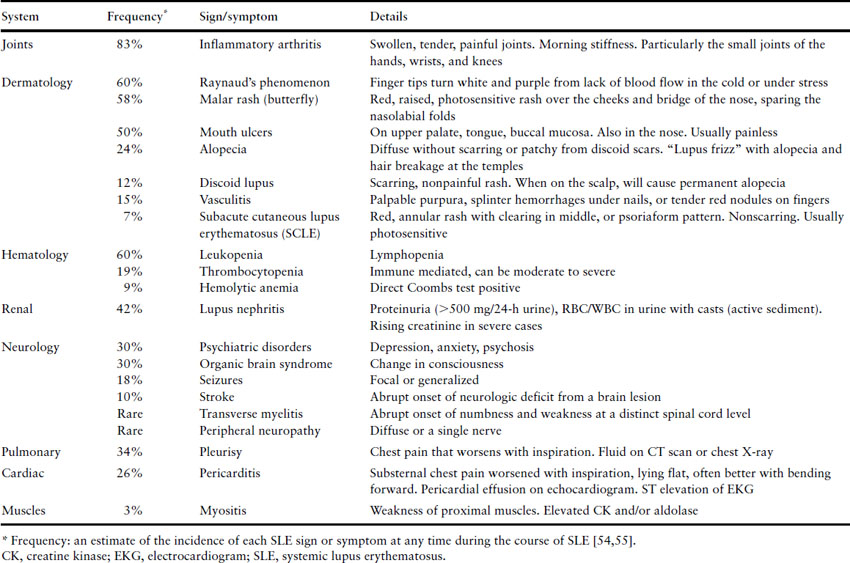
Systemic lupus erythematosus can present with myriad of symptoms, many of which are nonspecific (Table 8.2). The most common symptoms include fatigue, arthralgias, especially in the small joints of the hands, rashes, low-grade fevers, Raynaud’s phenomenon and hair loss.
Table 8.2 Signs and symptoms of active lupus
Box 8.2 ACR revised classification criteria for SLE. A patient must have at least 4/11 to be labeled with SLE. The ANA will be positive in over 95% of women with SLE
- Malar rash
- Discoid lupus
- Photosensitive rash
- Mouth ulcers
- Arthritis
- Serositis (≥1):
- pericarditis
- pleurisy
- pericarditis
- Nephritis (≥1):
- Proteinuria ≥3+ or ≥0.5 g per 24 h
- Cellular casts in urine
- Proteinuria ≥3+ or ≥0.5 g per 24 h
- CNS lupus (≥1):
- seizures
- psychosis
- seizures
- Hematologic abnormalities (≥1):
- Leukopenia/lymphopenia
- Hemolytic anemia
- Thrombocytopenia
- Leukopenia/lymphopenia
- Other antibodies (≥1):
- anti-dsDNA antibody
- Sm (Smith) antibody
- anticardiolipin antibodies
- lupus anticoagulant
- anti-dsDNA antibody
- Positive ANA
ANA, antinuclear antibody; CNS, central nervous system; SLE, systemic lupus erythematosus.
Laboratory findings in lupus
The ANA is the classic autoantibody associated with SLE. It is present in over 95% of patients with SLE, but can also be found in up to 25% of the general population and patients with other autoimmune disease [16]. Therefore, finding a positive ANA, even at a high titer, does not clinch a diagnosis of SLE. Though the ANA titer may fluctuate over time, this fluctuation has no relationship to disease activity or likelihood of an SLE diagnosis. Therefore, once a high-titer ANA has been identified (i.e. ≥1:320), there is no reason to repeat this laboratory test. Similarly, a rising ANA titer should not be a cause for alarm.
The ANA is a nonspecific antibody that can be further classified by the specific antigen that it targets. These specific targets include the other antibodies used to define SLE and other connective tissue diseases (Table 8.3). The double-stranded DNA and Sm antibodies are unique to lupus, and the vast majority of patients with these antibodies will carry this diagnosis. The dsDNA antibody titer can fluctuate with disease activity, and therefore it is frequently monitored in patients with SLE. The Sm, Ro, La and RNP antibodies do not fluctuate in a meaningful way. Once these have been identified as positive, they need not be re-evaluated. The Ro and La antibodies are important in pregnancy because they can cause neonatal lupus. For this reason, we recommend checking these antibodies at the onset of pregnancy, even if previously negative, to help guide neonatal evaluation (see neonatal SLE discussion below).
A significant minority of women with SLE will have hematologic abnormalities. Thrombocytopenia, prompted by antiplatelet antibodies, can drive platelets to dangerously low levels. A mild chronic anemia is fairly common, but Coombs positive hemolytic anemia can also occur. Many women with SLE will have a mild lymphopenia and leukopenia. This low white blood cell count is rarely a sign of true immunosuppression.
Lupus nephritis is a common, and potentially devastating, component of SLE. Over half of all women with SLE will develop nephritis at some point, but fortunately many cases are mild. Lupus nephritis is best identified through the urinalysis. The urine will have elevated protein (>500 mg/24-h collection) and an “active sediment,” meaning increased red and white blood cells and, potentially, cellular casts. Early on, lupus nephritis is asymptomatic, so routine urinalysis is important. However, symptoms such as severe edema from low albumin or signs of uremia may be presentations of more severe disease.
Differential diagnosis
As the symptoms, signs and laboratory testing for SLE can be nonspecific, the differential diagnosis is wide. For women with a positive ANA, other connective tissue diseases must be considered (see Table 8.3). Hematologic abnormalities and lymphadenopathy may indicate a malignancy. Alternative causes for nephritis must be considered and a renal biopsy is indicated in most women with significant persistent proteinuria or worsening renal function.
Table 8.3 The connective tissue diseases
Lupus during pregnancy
Most women with SLE will have some disease activity during pregnancy. Fortunately, however, in the vast majority of patients this activity will be mild, predominantly arthritis and cutaneous disease. A minority of women (up to 30%) will have more significant disease activity [17]. Lupus can flare at any time during pregnancy, including in the weeks post partum. The factors that best predict quiet lupus in pregnancy are:
- quiet lupus activity in the 6 months prior to conception
- few severe flares in the years prior to pregnancy
- continuation of hydroxychloroquine (Plaquenil) through pregnancy.
Fetal complications in SLE pregnancies
The overall pregnancy loss rate is higher among women with SLE. One study of women with SLE compared to their sisters and friends found that the pregnancy loss rate was 2–3-fold higher with SLE [18]. A population-based study of women with SLE found that the pregnancy loss rate prior to an SLE diagnosis was 15.2% and after an SLE diagnosis was 23.4%, both higher than the 8.3% loss rate in the general population. This is a lower estimate of miscarriage in the general population than is seen in most studies but does suggest an increased risk among women who either have or are developing SLE [19]. Cohort studies of SLE pregnancies demonstrate that the miscarriage rate (<20 weeks gestation) may be similar to the general population, but the stillbirth rate is significantly elevated. In the Hopkins lupus cohort, 8% of all pregnancies resulted in a stillbirth [18]. Risk factors for pregnancy loss are increased lupus activity and antiphospholipid syndrome (APS). Women with first-trimester activity, measured by thrombocytopenia, proteinuria or physician assessment, are at a threefold increased risk for pregnancy loss. Women with uncontrolled first-trimester hypertension are also at an increased risk for pregnancy loss [20].
On average, 33% of lupus pregnancies will result in a preterm birth [21]. Fortunately, most of these will be delivered after 28 weeks. Some deliveries are induced due to the health of the mother or fetus. However, most are spontaneous deliveries precipitated by preterm rupture of membranes or preterm labor [21]. Risk factors for preterm birth include increased lupus activity just prior to or during pregnancy, APS, use of high-dose corticosteroids, low complement and hypertension [17,21,22].
Systemic lupus erythematosus is associated with poor placentation. Placentas have more infarcts and perivillous inflammation, resulting in slowed growth among some infants [23]. In the Hopkins lupus cohort, over 20% of infants were below the 10th percentile of weight for gestational age [18].
Pre-eclampsia and SLE
Pre-eclampsia may be diagnosed in up to one-quarter of SLE pregnancies. The combination of hypertension and proteinuria in a woman with SLE, however, cannot automatically be assumed to be pre-eclampsia. Lupus nephritis, one of the most severe manifestations of SLE, presents in the same manner (Table 8.4). The presence of other SLE symptoms, such as fevers, rashes or arthritis, may assist in the diagnosis of an SLE flare. Treatment for lupus nephritis includes high-dose corticosteroids and immunosuppression. If left untreated, lupus nephritis can progress to renal failure and require dialysis support.
Table 8.4 Factors that distinguish between pre-eclampsia and SLE activity
Reproduced with permission from Clowse ME. Lupus activity in pregnancy. Rheum Dis Clin North Am 2007;33(2):237–52.
| Pre-eclampsia | SLE activity | |
| Risk factors | ||
| First pregnancy | Increases risk | No impact |
| Pre-eclampsia in prior pregnancy | Increases risk | No impact |
| Multifetal gestation | Increases risk | Unknown impact |
| History of lupus nephritis | Increases risk | Increases risk |
| Timing in pregnancy | Always after 20 weeks, usually after 30 weeks gestation | Anytime in pregnancy |
| Laboratory tests | ||
| Active urine sediment (WBC, RBC, casts) | Usually negative | Positive |
| Coombs test | Usually negative | May be positive |
| Antiplatelet antibody | Usually negative | May be positive |
| Complement (C3 and C4) | Usually normal | May be low |
| Anti-dsDNA antibody | Usually negative | May be positive |
| Serum uric acid | Over 5.5 mg/dL | No change |
| Urine calcium | Low | Normal |
| PlGF∗ | Low | Unknown |
| sFlt-1∗ | High | Unknown |
| Physical findings | ||
| Dermatologic disease | Not present | May be present |
| Arthritis | Not present | May be present |
| Serositis | Not present | May be present |
| ∗sFlt-1 and PIGF are currently under development and are not available outside research laboratories. | ||
| PlGF, placental growth factor; RBC, red blood cells; sFlt-1, soluble FMS-like tyrosine kinase 1; WBC, white blood cells. | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


