Introduction
Neurologic conditions are an important cause of morbidity and mortality in pregnant women. In the UK between 2003 and 2005, women with neurologic conditions accounted for 37 out of a total 87 maternal deaths, with stroke (n=24) and epilepsy (n=11) [1] accounting for the majority of deaths from neurologic causes [2]. Several other disabling neurologic conditions, ranging from multiple sclerosis to migraine, present both medical and obstetric challenges to clinicians unfamiliar with their management within the context of pregnancy.
The natural history of key neurologic conditions has been observed during pregnancy, providing a useful basis on which to counsel women and organize services. Less information is available about the safety of neurologic treatments or investigations during pregnancy or obstetric and neonatal outcomes for these women. Therefore many decisions rely on applying key obstetric and neurologic principles to situations where published data are not available.
This chapter outlines neurologic assessment and investigation in pregnancy. The most common and important neurologic presentations – multiple sclerosis, epilepsy, stroke, central nervous system (CNS) tumors and headache – are considered. These disparate conditions allow basic principles of management to be described. Overall, women with pre-existing neurologic conditions or who develop neurologic symptoms during pregnancy rely on good communication between their obstetrician, neurologist, physician and anesthetist to achieve optimal obstetric and neonatal outcome.
History and examination
Neurologic symptoms in pregnancy should be elicited in the usual way with particular emphasis on their onset and progression, and their associated features.
Higher mental function
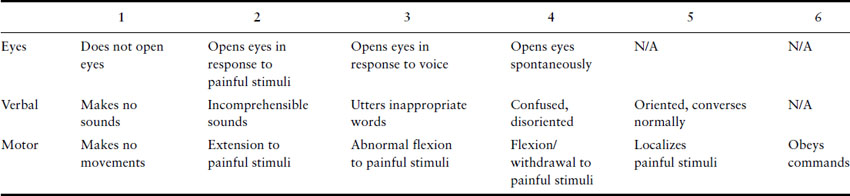
Neurologic examination should first consider higher mental function. It may be sufficient to establish that the patient is alert and orientated, and that her conversation is normal in both its form and content. But any suspicion of impaired cognitive functioning either at the time of assessment or in the past should lead to assessment of conscious level using a simple instrument such as the Glasgow Coma Scale (Table 15.1) or the more detailed Mini-Mental State Examination (Box 15.1).
Table 15.1 Glasgow Coma Scale
Cranial nerves, trunk and limbs
Neurologic examination of the cranial nerves, trunk and limbs can be an involved and lengthy process. Over 150 years, numerous eponymous signs and methods of examination have been described but what follows is a basic neurologic screen, which should be sufficient for an obstetrician to establish most important neurologic findings, which will guide investigation and further referral.
The minimum cranial nerve examination involves consideration of each of the 12 cranial nerves (Table 15.2).
Table 15.2 Examination of cranial nerves – summary
Trunk and limbs
Examination of the trunk and limbs involves examination of their appearance (inspection), tone, strength, co-ordination and sensory perception.
Box 15.1 Mini-Mental State Exam
The Mini-Mental State Examination (MMSE) is a tool that can be used to objectively assess mental status. It is an 11-question measure that tests five areas of cognitive function: orientation, registration, attention and calculation, recall, and language. The maximum score is 30. A score of 23 or lower is indicative of cognitive impairment. The MMSE takes only 5 minutes to administer and is therefore practical for routine repeated use.
Orientation
5 ( ) What is the (year) (season) (date) (day) (month)?
5 ( ) Where are we (state) (country) (town) (hospital) (floor)?
Registration
3 ( ) Name 3 objects: 1 second to say each. Then ask the patient
all 3 after you have said them. Give 1 point for each correct answer.
Then repeat them until he/she learns all 3. Count trials and record.
Attention and calculation
5 ( ) Serial 7’s. 1 point for each correct answer. Stop after 5 answers.
Alternatively spell “world” backward.
Recall
3 ( ) Ask for the 3 objects repeated above. Give 1 point for each correct answer.
Language
2 ( ) Name a pencil and watch.
1 ( ) Repeat the following “No ifs, ands, or buts.”
3 ( ) Follow a three-stage command: “Take a paper in your hand, fold it in half, and put it on the floor.”
1 ( ) Read and obey the following: CLOSE YOUR EYES.
1 ( ) Write a sentence.
1 ( ) Copy the design shown.
Adapted from: Folstein M Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12(3):189–98.
Inspection involves looking for atrophy, hypertrophy, spasm or fasciculations of the muscles.
Tone can be examined throughout range of movement in each of the major joints and it may be appropriate to assess truncal tone, either in the sitting or ideally the standing position. In general, increased tone may be the result of a CNS disorder affecting the brain or spinal cord whereas reduced tone (flaccid weakness) is the result of a more peripheral problem.
Power is described by the Medical Research Council (MRC) scale (0–5) at each major joint in flexion, extension and if necessary in abduction and adduction (Box 15.2).
The pattern of any weakness demonstrated is important; for example, metabolic disorders such as thyroid disease or diabetes may cause a more proximal weakness. Neuropathies typically cause a distal weakness.
Co-ordination can be assessed in the upper limbs by examining ability to alternately touch the examiner’s outstretched finger and then the patient’s own nose. A further examination is for the presence of dysdiadochokinesis, which is assessed by asking the patient to rapidly tap their palm with alternating sides of their fingers, and considering the fluency and speed with which this is achieved. Assessment of lower limb co-ordination involves examination of normal gait, and then tandem (heel to toe) walking.
Sensation should be assessed using a “sharp” stimulus (ideally a “Neurotip”, although a broken tongue blade may be used if nothing else is available) and dabbing (rather than stroking) with cotton wool or similar stimulus. Thought should be given to the pattern of sensory loss expected from history and earlier examination. For example, a woman complaining of back pain may have radicular (nerve root) rather than distal (“glove and stocking”) sensory loss. Even where there is no obvious loss of sensation to pinprick or soft touch, joint position and vibration sense may be impaired.
Box 15.2 MRC scale for muscle power
| 0 | No muscle contraction is visible |
| 1 | Muscle contraction is visible but there is no movement of the joint |
| 2 | Active joint movement is possible with gravity eliminated |
| 3 | Movement can overcome gravity but not resistance from the examiner |
| 4 | The muscle group can overcome gravity and move against some resistance from the examiner |
| 5 | Full and normal power against resistance |
Reflexes and plantar responses (reviewed in Boxes 15.3 and 15.4) are an important element of both motor and sensory examination. Brisk reflexes are very common in pregnancy but may indicate hyperarousal or CNS pathology – and the latter is supported if the plantar reflexes are extensor. Clonus (five or more repetitive, rhythmic contractions of a muscle when attempting to hold it in a stretched state) is always abnormal. Reduced or absent reflexes may imply a neuropathy.
Finally, Romberg’s test, where the patient is asked to stand with their feet close together and eyes closed, is sensitive to a wide range of neurologic impairments. For example, patients with pathologies as disparate as distal neuropathy, cerebellar disease or generalized weakness may all sway when the test is performed, and this can act as a useful screening check that an abnormality has not been missed in an otherwise normal patient.
Neurologic investigations
Many neurologic investigations pose no risk to the pregnant mother or her fetus. For example, neurophysiologic assessment such as electroencephalography (EEG), nerve conduction studies and electromyography (EMG) may be uncomfortable but are noninvasive.
Box 15.3 Deep tendon reflexes and the associated spinal cord nerve roots
- Biceps reflex (C5, C6)
- Brachioradialis reflex (C5, C6, C7)
- Triceps reflex (C6, C7, C8>)
- Patellar reflex or knee-jerk reflex (L2, L3, L4)
- Ankle jerk reflex (Achilles reflex) (S1, S2)
- Plantar reflex or Babinski reflex (L5, S1, S2)
Box 15.4 The plantar reflex
The lateral side of the sole of the foot is rubbed with a blunt instrument from the heel along the curve to the metatarsal pads of the toes.
A “flexor response” (absent Babinski response), in which the toes curl into themselves, is normal.
An “extensor response” (the Babinski reponse is present), in which the big toe bends up towards the top of the foot and the other toes fan out, is abnormal and is highly suggestive of central nervous system damage.
Cerebrospinal fluid (CSF) is obtained by lumbar puncture and can show evidence of intrathecal infection, inflammation or the presence of subarachnoid hemorrhage or neoplasia. Lumbar puncture is relatively invasive and standard relative and absolute contraindications should be considered. In particular, lumbar puncture may cause CSF leak and a fall in CSF pressure. Neuroimaging should precede this investigation if raised intracranial pressure is suspected. Interpretation of CSF analysis is reviewed in Chapter 16.
Neuroimaging is often an integral part of patient assessment. Diagnostic imaging in pregnancy is reviewed in Chapter 32 but will be briefly reviewed here. The ionizing radiation associated with computed tomography (CT) neuroimaging leads to obvious concern on the parts of both clinicians and patients when imaging the pregnant woman. However, CT head involves a fetal radiation exposure of less than 0.005 mGy (0.0005 rad) and thus is extremely unlikely to have any fetal effects [3]. Uterine shields are routinely employed and will reduce exposure even further. The advantages that CT imaging of the head offers by way of early diagnosis, particularly when cerebral hemorrhage is suspected, should outweigh any cultural aversion to radiologic imaging in pregnancy.
Magnetic resonance imaging (MRI) of the brain has greater resolution than CT and greater diagnostic flexibility. It does not involve ionizing radiation and thus avoids any issues related to radiation exposure in pregnancy. However, although there is no evidence of fetal harm in humans after 20 years of widespread use of this technology, many guidelines recommend that MRI be delayed to the end of the first trimester when possible. Two other disadvantages of MRI are that performing the scans takes up to 45 minutes and they are more difficult to obtain in a timely manner in most countries as there are fewer MR than CT scanners.
Iodinated contrast agents can be used in pregnancy and lactation but intravenous contrast agents used in MR (e.g. gadolinium) are not [4].
Certain clinical situations may indicate the use of other ionizing radiologic techniques such as digital subtraction angiography. The expected radiation dose depends on the procedure. For example, this may be low with head imaging, but higher for spinal procedures. Clinicians should balance the risks of not fully investigating a patient against potential harm to the fetus.
Definition and Incidence
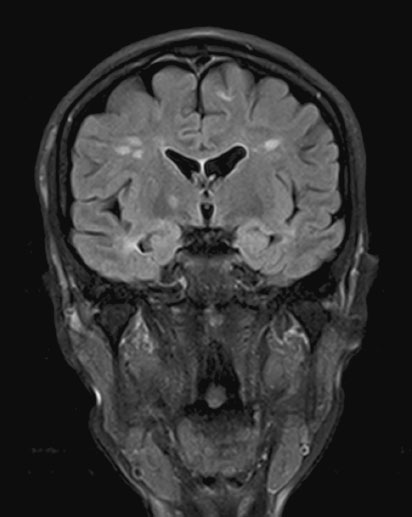
Multiple sclerosis (MS) is a disease of the CNS (brain and spinal cord) that is characterized by both neuroinflammation and neurodegeneration (Figure 15.1). Incidence is 3.6 cases per 100,000 person-years (95% confidence interval (CI) 3.0–4.2) in women and is higher in northern latitudes, although this trend seems to be reducing [5]. The disease can have many clinical manifestations (with sensory loss in the limbs, visual loss, subacute motor loss, double vision and gait disturbance being most common) and a highly variable pace of progression (relapsing remitting, secondary progressive, primary progressive and progressive relapsing). The median survival from onset of symptoms is 38 years. MS is usually diagnosed between 20 and 50 years of age, and women with MS will therefore become pregnant relatively early in the course of their illness and have correspondingly little associated disability [6]. Nevertheless the issues of symptom management and counseling are relevant at all stages throughout pregnancy.
Preconception advice
Preconception counseling offers the opportunity to discuss the effect that pregnancy may have on MS, the effect that MS-related neurologic impairment may have on pregnancy and delivery, and the use of disease-modifying or symptomatic treatments both in pregnancy and post partum.
Many people with relapsing and remitting MS are treated with disease-modifying drugs (DMD). These include beta-interferons and a synthetic polypeptide, glatiramer acetate. These treatments are administered by subcutaneous or intramuscular injection at least weekly and have been shown to have a modest but significant effect on reducing relapse rate (approximately 30% per year) and to some extent long-term disability. The safety of these treatments in pregnancy has not been established. The US Food and Drug Administration (FDA) has assigned glatiramer acetate to pregnancy safety category B (i.e. appears to be safe for pregnancy in animal studies but not adequately studied in pregnant humans). It has not been shown to be a teratogen in animal studies and its large molecular weight (4700–11,000) suggests that if it crosses the placenta, it does not do so by simple diffusion. The interferons are US FDA pregnancy safety category C (i.e. human studies are lacking and animal studies are either positive for fetal risk or lacking as well). In high doses, interferons appear to be abortifacients, but not teratogens. Limited human data suggest that interferon-alpha (not used to treat MS) does not cross the placenta or cause congenital anomalies in humans. However, data on interferon-beta products used to treat MS are very limited and suggest it may be an abortifacient [7].
The authors recommend that women should therefore stop treatment with DMD if they are planning to become pregnant or find themselves pregnant. This is less because of any known toxicity of the majority of DMD and more because of the combined considerations of the limited human pregnancy safety data and the fact that many of these agents have only limited efficacy over long periods of time. Although stopping treatment may expose the patient to increased risk of relapse, in absolute terms the risk is relatively low (an “extra” 0.2 relapse/year) [8]. This risk is further modified by the beneficial effect of pregnancy itself (see below). Other experts would offer women with MS the option of continuing agents for which there is reassuring pregnancy data such as glatiramer acetate.
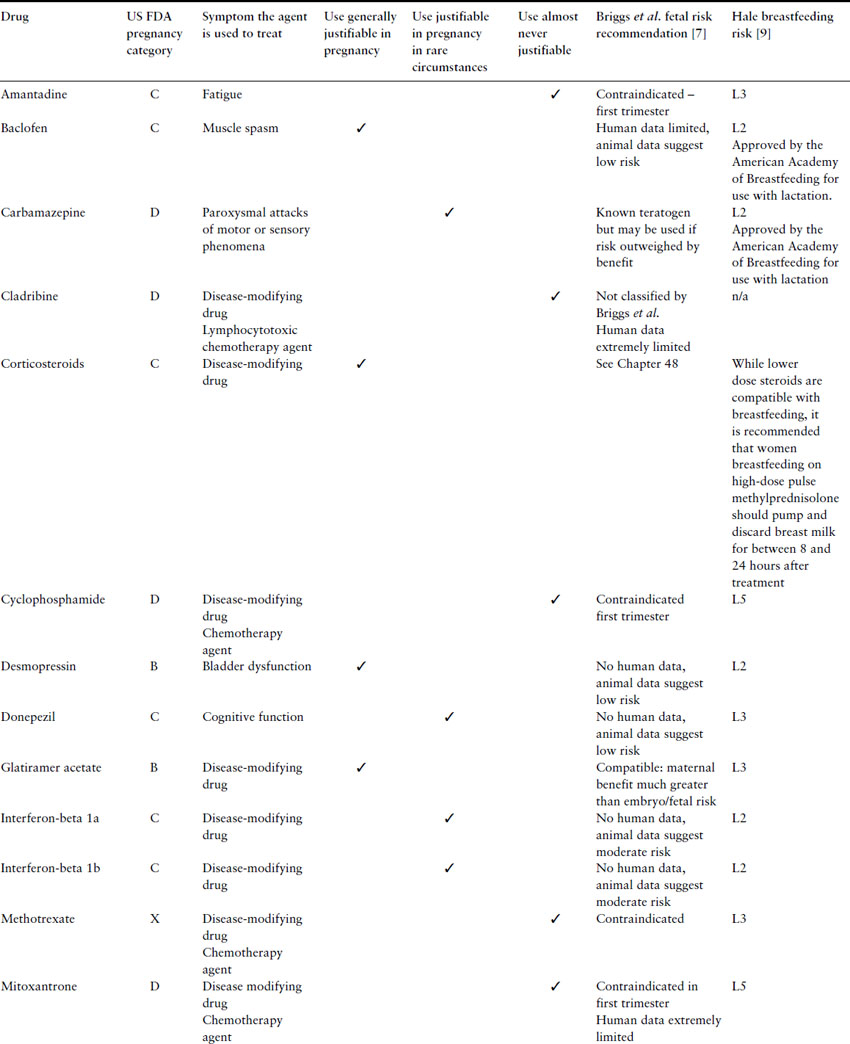
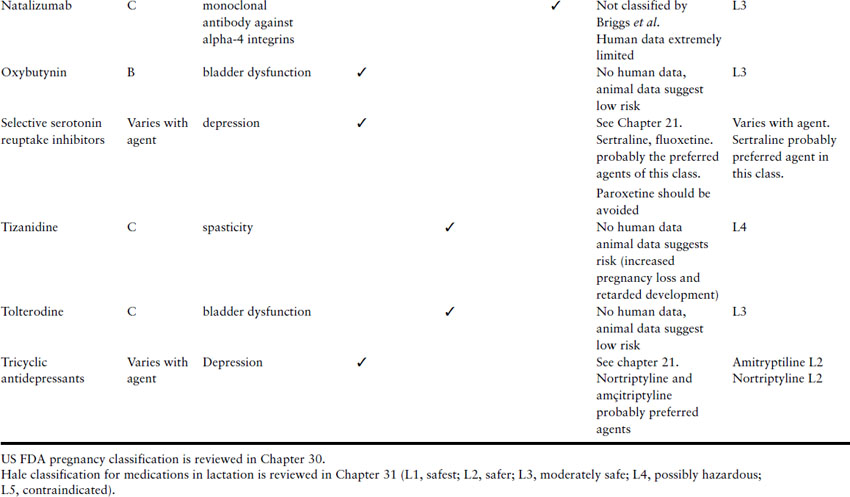
Women with MS may also be taking other drugs, such as antimuscarinics for bladder disorders (e.g. oxybutinin), antispasmodics (e.g. baclofen or diazepam), and antidepressants (e.g. tricyclic antidepressants). All these have low teratogenic potential. Table 15.3 lists some agents used to treat patients with MS and offers some opinions as to which agents might be considered for continued use or introduction during pregnancy.
Table 15.3 Risks and recommendations about the use of agents to treat MS during pregnancy
Rarely, patients with MS or other conditions that cause spasticity have implantable devices delivering an antispasmodic agent, typically intrathecally. These pumps are sited within the abdominal wall, extraperitoneally. They supply a continuous infusion of baclofen through a catheter directly into the CSF in small doses. Several case reports have reported successful obstetric outcomes using these devices [8a].
The inheritance of MS is poorly understood and is likely to involve a complex interaction between genetic and environmental factors. Nevertheless, in general the child of a mother with MS has an approximately 2–3% chance of developing MS, compared with 0.1% prevalence in the general population [10] although the mother should be counseled that should MS occur, symptoms are unlikely to present until their third decade or later. The risk is increased further if both parents are affected.
Effect of pregnancy on relapse rate
One of the most significant issues to discuss with women who are planning to become pregnant is the likely effect of pregnancy on relapse rate. In the past, the relatively high number of relapses observed post partum led to the false conclusion that pregnancy might lead to a long-term poorer outcome when compared with nonpregnant women. More reassuring advice should now be given, largely through the publication of a large prospective study (“PRegnancy In MS” (PRIMS)), which has continued to monitor enrolled mothers many years after enrolment into the study [11]. This study showed that although risk of relapse post partum was increased for approximately 3 months by a factor of two, this was equally balanced by the observation of significantly fewer relapses during pregnancy. Thus, overall no difference is observed in the long-term disability of women who become pregnant.
Relapse management
Although the risk of relapse is reduced during pregnancy, this effect is less pronounced during the first and second trimesters. Should relapse occur, management is the same as for nonpregnant women. Mild relapses require no treatment, but are likely to warrant assessment by occupational or physiotherapists, as levels of disability may be increased for several weeks. High-dose corticosteroids (for example, a total of 3 g methylprednisolone administered over 3–5 days intravenously or orally) may be used to speed up remission. Steroids present well-known risks including reduced bone density, infection, mood alteration and adverse gastrointestinal effects. Nevertheless, where standard precautions and pretreatment assessment are applied, steroids may avoid the need for hospitalization or reduce considerably the length of stay. The risks of glucocorticoid use for the embryo and fetus are discussed in Chapter 48 but these agents can and should be used when indicated to treat MS during pregnancy.
Occasionally, relapses are severe and progressive. In the nonpregnant woman these may be treated with more aggressive treatment, such as mitoxantrone (a chemotherapeutic agent) or natalizumab (a monoclonal antibody directed against alpha-4-integrin, which reduces white cell traffic into the CNS). These treatments are either clearly teratogenic and embryotoxic or have no evidence to support their safety in pregnancy. Clinicians should consider the advantages and risks to both mother and fetus on a case-by-case basis. Intravenous immunoglobulin [12] or possibly hemodialysis are alternative treatments, which may pose less risk to the fetus, but there is less evidence to suggest their efficacy in reducing MS progression.
Management of other symptoms
Women with MS may suffer from a range of symptoms during pregnancy including fatigue, restless lower limbs and urinary symptoms (see Table 15.3).
Nonpharmacologic advice to improve quality of sleep (“sleep hygiene”) should be offered. This includes examination of an individual’s sleep routine and the sleeping environment. Women should be encouraged to avoid psychologically stimulating activities in the evenings and pharmacologic stimulants such as caffeine should be minimized. Drug treatments such as amantadine or modafanil, which are sometimes used to reduce MS-related fatigue, cannot be recommended, as there is no evidence to support their safety in pregnancy.
A sense of restlessness in the lower limbs is common in pregnant women, but women with MS may also have a degree of spasticity manifest by painful or irritating spasm. Neurologic examination can be useful to demonstrate spasticity and localize relevant muscle groups. Physiotherapy advice and possibly use of benzodiazepines may be warranted.
Urinary symptoms should be carefully evaluated as impaired bladder emptying in women with MS predisposes to infection. Baclofen to treat bladder spasm is a reasonable option in pregnancy. Vigilance for early signs of urinary tract infections is important for any pregnant woman with bladder dysfunction. Women with pre-existing urinary problems may be concerned that vaginal delivery will exacerbate urinary symptoms post partum and may benefit from some discussion with their obstetrician as to the relationship between mode of delivery and future urinary continence.
Third trimester and delivery
In most cases, third trimester and the peripartum period are no different between populations of women with MS and the normal population. Specifically, obstetric and neonatal outcomes do not differ, with similar rates of induction, instrumentation, cesarean section and infant mortality.
Nevertheless, women with MS may have specific neurologic impairments that affect interpretation of symptoms during pregnancy. For example, women with plaques at T11 or lower will have impaired bladder and bowel function but normal sensation of uterine contractions and pain. Lesions between T6 and T10 will impair perception of uterine contractions and where significant lesions are present above T6, other signs of labor will have to be considered such as worsening lower limb spasticity.
In the past theoretical concerns have been expressed regarding the safety of epidural anesthesia and exacerbation of MS. Evidence from the PRIMS study, however, has been reassuring, demonstrating no significant difference in outcomes between women with and without epidural anesthesia.
Post partum
Women who are more mildly affected by MS are more likely to choose to breastfeed. Breastfeeding does not protect (or cause) a post partum relapse [13]. Women who have been taking DMD before pregnancy balance the benefits of breastfeeding against the risk of relapse without treatment. The agents commonly used to treat MS are classified according to their compatibility with breastfeeding in Table 15.3. Women who defer DMD treatment should be counseled that should they suffer relapse, most centers require good recovery before DMD are restarted, thus producing further delay.
Some centers have advocated prophylactic use of intravenous immunoglobulin post partum to prevent relapses [14]. Activity of disease in the year before pregnancy and in the first trimester to some extent predicts risk of relapse in the 3 months post partum. Nevertheless, a recent authoritative multivariate model predicts that using this information to make a decision about treatment would lead to 50% of women being treated unnecessarily [13] and the current consensus is therefore not to treat.
Definition and incidence
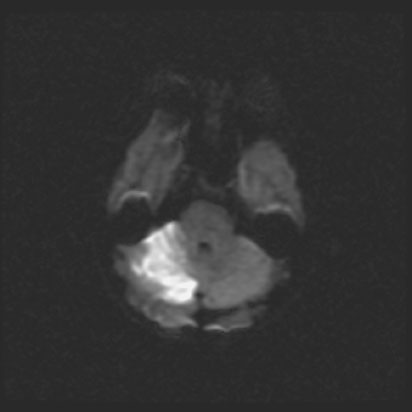
Stroke is an acute neurologic impairment that follows interruption of blood supply to a specific part or the brain (Figure 15.2). It is the cause of 700,000 deaths per year in the US, a total of 1 in 15 of all deaths. The causes of stroke in the general population are reviewed in Box 15.5.
Box 15.5 Causes of stroke in the nonpregnant population
Ischemic 85%
- Thrombosis
- Arterial
- Venous
- Embolism
- Venous
- Hypoperfusion
Hemorrhagic 15%
- Intracerebral, usually hypertension related
- Subarachnoid hemorrhage, usually related to aneurysms and AVM
Stroke is an uncommon but serious complication of pregnancy. The incidence of stroke in nonpregnant women aged 15–44 has been reported to be as low as 10.7 per 100,000 women-years [15]. Multicenter or long-term observational studies are therefore required to establish incidence in pregnancy. Estimates using such methods produce widely differing rates between 4.3 and 210 strokes per 100,000 deliveries, depending on inclusion criteria, with most studies suggesting an increased risk of stroke associated with pregnancy [16–18]. Most (up to 90%) strokes in these studies occurred at the time of delivery or or in the weeks following [19].
Risk factors for stroke
Physiologic
Progressive physiologic changes occurring throughout pregnancy predispose to stroke, including increasing hypercoagulability, venous stasis and vascular wall changes. The second stage of labor involves episodes of significantly increased intrathoracic pressure (Valsalva) and elevation of cerebral perfusion pressure, which may lead to changes in cerebral blood flow, particularly where cerebral autoregulation or anatomy is disordered [20].
Obstetric
The main obstetric factor associated with an increased risk of stroke is pre-eclampsia, in particular uncontrolled systolic hypertension. This is still the major cause of death due to pre-eclampsia in the UK [1]. Age more than 35 years, black ethnicity, greater parity and multiple gestation are all risk factors for stroke although quantifying this risk is not possible from available data.
Co-morbidity
Women who become pregnant may have co-morbidity that increases their risk of vascular events, including stroke, such as obesity (Body Mass Index (BMI) >30 kg/m2), diabetes, pre-existing hypertension, renal and heart disease, vasculopathies such as sickle cell disease, vasculitis and pre-existing collagen or atherosclerotic disease. Alcohol, tobacco and cocaine use may cause a vasculopathy or hypertension.
Migraine with aura (see later) also produces excess risk for stroke but this condition is common and stroke in pregnancy is rare, and so caution should be applied when counseling women about this risk factor.
Previous stroke during pregnancy presents a particular dilemma for women considering further pregnancy. Unfortunately, data are lacking although in a follow-up study, 13 of 489 (2.7%) women aged 15–40 who had suffered a stroke had a recurrent event and only two of these occurred during pregnancy [21]. Full ascertainment of vascular risk factors, including CT or MR angiography, is likely to be appropriate to best inform the individual of her likely risk of pregnancy-related recurrent stroke.
Clinical presentation and management of stroke
Presentation and investigation
Stroke presents as in the nonpregnant and clinical features may suggest either infarction or hemorrhage but neuroimaging is required to confirm diagnosis. Stroke should be considered in any woman who presents with any of the symptoms listed in Box 15.6. While an imperfect screen with a specificity of 88% and a sensitivity varying from 66–100%, the Cincinnati Prehospital Stroke Scale may be useful in obstetric patients presenting with headache or other softer neurologic complaints to help decide if a patient warrants prompt complete neurologic assessment and neuroimaging. This screening test is summarized in Box 15.7.
As stated above, most pregnancy-related cerebral infarction occurs in the puerperium [17,23,24] at a time when the mother is often bedbound, still hypercoagulable and may just have had pelvic surgery. Widespread adoption of postpartum thromboprophylaxis in the UK has been associated with a decrease in the incidence of cerebral infarction due to emboli, but not that due to uncontrolled systolic hypertension [1].
Box 15.6 Symptoms that warrant consideration of stroke
- Sudden weakness or numbness of face, arm or leg especially if on one side of the body
- Sudden confusion
- Trouble speaking or understanding
- Sudden trouble seeing in one or both eyes without a prior history of migraines
- Sudden trouble walking
- Sudden loss of balance or co-ordination not readily attributable to pregnancy
- Sudden severe headache with no known cause
Box 15.7 Cincinnati Stroke Scale [22]
Facial droop
- Have the patient smile and assess for facial droop
- Normal: both sides of face move equally
- Abnormal: one side of face does not move
Arm drift
- Have the patient hold both arms out and up with palms facing upwards
- Normal: both arms move equally
- Abnormal: one arm drifts compared with the other
Speech
- Have the patient repeat a sentence
- Normal: patient uses correct words with no slurring
- Abnormal: slurred or inappropriate words of mute
If any of these three elements or any other neurologic findings are newly abnormal, the possibility of acute stroke is high and the patient should have urgent imaging and evaluation by a neurologist.
Stroke is a medical emergency. Patients with acute arterial ischemic stroke from embolism or thrombosis can have their long-term outcome greatly improved by the use of thrombolytic therapy within 180 minutes of the onset of symptoms. Therefore all patients with symptoms suggestive of stroke require prompt neuroimaging to determine if they have had an ischemic stroke that may benefit from the use of thrombolytic therapy. Box 15.8 lists the recommended guidelines for timing of interventions for patients presenting with acute ischemic stroke. Box 15.9 reviews the other assessments recommended for patients presenting with possible acute stroke. Patients should be positioned with the head of the bed between 0º and 15º and blood pressure should be treated acutely if greater than 180/105 with labetalol. Aspirin should not be given while investigating an acute stroke. Box 15.10 reviews the contraindications for thrombolytic therapy. The use of thrombolysis should be considered for pregnant and postpartum women with severe acute cerebral nonhemmorhagic infarction if it can administered within 180 minutes of onset of the neurologic deficit [25]. Thrombolysis is well tolerated by the fetus in pregnancy and should not be withheld if the maternal condition is life-threatening. Risks of postpartum uterine or pelvic haemorrhage can be dealt with by local haemostasis or surgery.
The authors and editors would, however, not recommend the use of thrombolytics for acute ischemic stroke in the setting of probable or confirmed pre-eclampsia.
Box 15.8 Guidelines for interventions in acute ischemic stroke
Patients presenting with symptoms of acute stroke to an emergency room should:
- be seen by a provider within 10 minutes with early notification of local “stroke team” of possible stroke patient
- have a neurologic assessment and head CT performed within 25 minutes of presentation
- have the head CT scan read within 45 minutes of presentation to determine whether they are candidates for fibrinolytic therapy
- receive fibrinolytic therapy administered within 60 minutes of presentation to the ER and no longer than 180 minutes since the time of onset of symptoms.
Box 15.9 Additional investigations for patients presenting with possible acute stroke
- Assess airway (can the patient protect her own airway or does she require intubation?), breathing (what is her respiratory rate and oxygenation?) and circulation (are her pulse and blood pressure normal?)
- Obtain secure intravenous access
- Obtain a fingerstick glucose
- Obtain a complete blood count, electrolytes, blood urea nitrogen, creatinine, serum glucose, serum troponin, liver function tests
- Consider urine toxicology screen and blood alcohol level
- Arterial blood gas if oxygen saturation abnormal Obtain an EKG
Specific syndromes will be considered below, but in most cases pregnant women who have cerebral infarction should be managed within a multidisciplinary stroke unit. Aspirin is the mainstay of treatment for acute ischemic sroke. Aspirin and the other anitplatelet agents (aspirin with dipyridamole, or clopidogrel) are also the most effective preventive treatment of stroke. Unfractionated or low molecular weight heparin is not recommended for acute stroke or stroke prevention except in the case of stroke from cardioembolism, arterial dissections or large artery intraluminal thrombus [26]. Warfarin is teratogenic and usually avoided in pregnancy.
Box 15.10 Contraindications and cautions to thrombolytic therapy for acute stroke
Contraindications
- Intracranial hemorrhage on CT
- Presentation suggests SAH
- Multilobar infarction on CT
- History of intracranial hemorrhage
- Uncontrolled hypertension (>185/110 when treatment with fibrinolytics to be given)
- Known AVM/neoplasm
- Witnessed seizure at onset of stroke
- Active bleeding/acute bleeding diathesis (platelets <100, PTT elevated, INR >1.7)
- Within 3 months of intracranial or intraspinal surgery/serious head trauma or previous stroke
- Arterial puncture at a noncompressible site in the past 7 days
Cautions: consider whether benefits of thrombolytic therapy outweigh risks
- Minor or clearing stroke
- Within 14 days of major surgery or trauma
- Within 21 days of GI/GU hemorrhage
- Within 3 months of acute MI
- Post MI pericarditis
- Glucose <50 or >400 mg/dL
The differential diagnosis of acute stroke in pregnancy is broad and includes migraine, transient ischemic attacks, head trauma, brain tumor, Todd’s palsy (a neurologic deficit following a seizure), systemic infection, functional deficits (“conversion disorders”), and toxic-metabolic disturbances (e.g. hypoglycemia, acute renal failure, hepatic insufficiency, drug intoxication). Perhaps the most challenging and common differential diagnosis for stroke in the obstetric population is migrainous aura. Migrainous auras are typically brief and more likely to be positive (the alteration of a sensory perception) rather than negative (the absence of a perception) (e.g. wavy lines in vision versus no vision or “pins and needles” versus numbness). Migrainous auras are most commonly visual (typically scotoma and/or zig-zag lines) or sensory (“pins and needles”) in the perioral region. Less commonly, they are sensory in the upper limbs or difficulties with speech (typically disarticulation with word-finding difficulty or use of wrong words but no difficulty with comprehension). Neurologic symptoms other than these should not be casually attributed to migrainous aura. Visual or sensory symptoms should be one-sided, gradually progress and last between 5 and 60 minutes. If more than one aura symptom is present, symptoms should occur in succession rather than simultaneously. Importantly, migraine and migrainous aura is by definition a recurring problem and the diagnosis cannot be made on first presentation of symptoms. If there is doubt about whether a patient’s symptoms represent stroke/transient ischemic attack or migrainous aura, an evaluation by a neurologist and neuroimaging is advisable [27].
Specific stroke syndromes and their management
Pre-eclampsia and eclampsia
Presentation
Pre-eclampsia is a multisystem disorder affecting 3–5% of pregnancies [28]. Although only a tiny proportion of those affected by pre-eclampsia suffer from stroke, up to 45% of women who have pregnancy-related stroke have pre-eclampsia or eclampsia [17,29]. Uncontrolled systolic hypertension and endothelial dysfunction may lead to hemorrhage or infarction [30]. Disordered cerebral autoregulation may also play a role, especially post partum.
Investigation and management
Investigation and management of pre-eclampsia will be familiar to the obstetrician and are reviewed in Chapter 5. Most cases of stroke in the setting of pre-eclampsia are due to arterial hemorrhage but cases of acute arterial thrombosis also occur. While pre-eclamptic stroke is most likely in the setting of severe hypertension (>180/110), it can occur at blood pressures much lower than this and the acute change in blood pressure may be as important a factor as the absolute number [31]. The only definitive treatment for pre-eclampsia is delivery of the fetus and placenta.
Prompt neuroimaging should occur in pre-eclamptic women with sudden-onset (thunderclap) headache and/or any persistent neurologic deficit. Neurosurgical consultation should be sought if intracerebral blood is found on CT or MRI to guide the need for interventions to decrease intracranial pressure. Blood pressure is typically brought to a level of 160/90 (a mean arterial pressure of 110 mmHg) and not much lower as some degree of hypertension may be needed to maintain cerebral perfusion and prevent ischemia. The presence of an intracerebral hemorrhage will complicate options for obstetric anesthesia and anesthesiologists should be involved early in these cases.
Reversible cerebral vasoconstriction syndromes
Presentation
Reversible cerebral vasoconstriction syndrome (RCVS) is an under-recognized and often misdiagnosed syndrome characterized by a sudden, severe headache at onset seen in association with a neurologic deficit. It is caused by reversible vascular narrowing involving the circle of Willis and its immediate branches. RCVS can present in conjunction with hypertensive encephalopathy, pre-eclampsia, and reversible posterior leukoencephalopathy, physical exertion or bathing and it can occur in isolation [32]. Women may have had an uncomplicated pregnancy and present a few days after delivery with headache [33], cerebral irritation and neurologic deficit. Investigations demonstrate infarction and/or hemorrhage.
The differential diagnosis includes subarachnoid hemorrhage, migraine, arterial dissection, vasculitis and infection.
Investigation and treatment
Computed tomography, MR or catheter angiography may demonstrate multifocal segmental narrowing of the cerebral vessels, which resolves within 4–6 weeks. Spinal fluid should be normal, and this distinguishes this syndrome from subarachnoid hemorrhage.
Treatment is supportive, although vasodilators and steroids have been used.
Intracranial hemorrhage
Presentation
Most intracranial hemorrhage occurring during an otherwise normal pregnancy is the result of aneurysmal subarachnoid hemorrhage (SAH) and arteriovenous malformation (AVM). Intracranial arterial dissections are a much rarer etiology. Hypertension, smoking, alcohol and family history are all risk factors. The incidence of SAH from aneurysmal rupture is 3–11 per 100,000 pregnancies [17,29] but 50% of all aneurysmal rupture in women below 40 occurs in the context of pregnancy [34]. Cavernoma and other venous anomalies are a very infrequent cause of hemorrhagic stroke.
Presentation of intracranial hemorrhage is the same as in the nonpregnant woman. Symptoms are dominated by the sudden onset of headache, often described as “the worst headache of my life,” and this presentation should always prompt consideration of the diagnosis of subarachnoid hemorrhage. Meningeal irritation (due to blood spreading through the CSF), altered consciousness, collapse or vomiting at onset, and the absence of lateralizing neurologic findings are features that are characteristic of SAH but not universal.
Investigation and management
Computed tomography scan is very sensitive for SAH within the first 12 hours after the event but is less sensitive with smaller bleeds and as the days go by after the initial event. Lumbar puncture is recommended in patients with a history suggestive of SAH who have a normal CT scan, especially if more than a day has passed since the onset of their symptoms. The presence of xanthochromia in CSF is highly suggestive of a SAH but will not be present until 2–6 hours after the acute event.
Computed tomography offers advantages compared with MRI in ease of obtaining a study and in the past was viewed as better than MRI at identifying early hemorrhage. However, the use of FLAIR and T2 sequences with MRI may be as good or better than CT at identifying a SAH, and is better than CT at identifying a SAH in the days following the acute event.
Ruptured aneurysmal SAH may be complicated by rebleeding, with an associated mortality rate of 50–70% [35] and so monitoring and management of such patients should have a high priority. Four percent of patients will rebleed within 24 hours of initial bleed, with up to 20% occurring within the first month. Vasospasm, cerebral infarction, hydrocephalus, increased intracranial pressure, seizures and hyponatremia are other possible complications. Medical treatment usually involves intravenous fluids, bed rest, compression stockings, analgesia, laxatives and nimodipine 60 mg 4 hourly [36]. Medical management of SAH should be undertaken at or in close liaison with a neurosurgical center.
Once the diagnosis is established, the etiology for the SAH must be determined with cerebral angiography, CT angiography or MR angiography. While cerebral angiography remains the most sensitive test, it is rapidly being replaced by CT angiography due to the ease of testing and steadily improving technology. All of these tests can be safely performed in pregnant or postpartum women when necessary.
Definitive treatment usually involves endovascular coiling or surgical clipping and the timing of these interventions will be decided by the neurosurgeon. In most cases, treatment during pregnancy is justifiable [34,37–39]. Outside pregnancy, coiling is felt to produce better overall outcomes than clipping [40]. In pregnancy, the risks of periprocedure use of radiation, postprocedure anticoagulation, and postcoiling rupture in remaining aneurysm tissue are generally readily outweighed by the benefit of effective intervention. At the time of SAH, women with aneurysms may temporarily lack capacity to consider these issues but in any case, detailed discussion between the obstetrician, the neurosurgical team and the family, wherever possible, should occur.
Women who have had a previous aneurysm completely obliterated by clipping or coiling may consider vaginal delivery [41]. Use of epidural anesthesia is advised. Some recommend avoidance of spinal anesthesia in women in whom the aneurysm is not totally obliterated [42], based on the hypothesis that the decrease in intracranial pressure caused by dural tap could cause an increase in transmural pressure across the arterial wall, thus facilitating the rupture of a potential vascular malformation. But anesthetic input is required as this fall in pressure is likely to be preventable.
Treatment of unruptured aneurysm
In general, management of unruptured aneurysms should be the same as in the nonpregnant state. Management of unruptured aneurysm in the nonpregnant individual is guided by the International Study on Unruptured Intracranial Aneurysms (ISUIA) [43]. Although rupture of aneurysm is associated with significant mortality, treatment of aneurysms also carries risk. ISUIA data suggest that the risk of treating certain low-risk aneurysms (small (<7 mm), asymptomatic, stable, anterior artery aneurysms) may be greater than the risk of conservative “watching and waiting.” In common with many trials, pregnancy has not specifically been considered. After discussion with the patient, it may be felt appropriate to treat such aneurysms preconceptually or during pregnancy. While there are no data to guide management of patients with untreated aneurysms in labor and delivery, most clinicians would recommend early good pain control, keeping blood pressure less than 140/90 mmHg and limiting the second stage of labor. An untreated aneurysm is not, however, viewed as an indication for cesarean delivery.
Unruptured AVM
Presentation
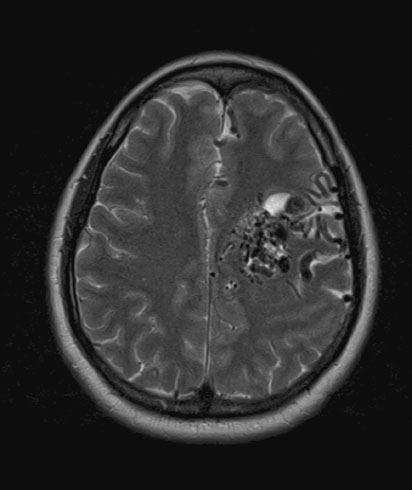
Arteriovenous malformations are less common than arterial aneurysms but present similarly with acute SAH. Unruptured AVM (Figure 15.3) present a lower bleeding risk than aneurysms, and overall risk of primary hemorrhage occurring during pregnancy is 3.5%, which is similar to the normal population [44]. Individual case reports suggest that pregnancy is not associated with significant changes to AVM [45], although the obstetrician or neurologist should emphasize the paucity of data to guide decisions in this area.
Treatment
Arteriovenous malformations are treated with combinations of surgery, endovascular embolization and stereotactic radiosurgery. The decision about treatment is guided by a number of factors including the site and complexity of the lesion.
In most cases, AVM are managed outside pregnancy. Although there is concern that untreated or partially treated AVM may be at risk of hemorrhage from the hemodynamic changes of labor, observed risk of hemorrhage is recognized to be low, particularly where epidural analgesia is used and second stage is assisted [46,47].
Cerebral venous thrombosis
Presentation
Cerebral venous thrombosis (CVT) may account for approximately 20% of strokes during pregnancy [24] and should be considered in any patient complaining of headache and drowsiness, particularly if focal neurologic signs or seizures are present. Its occurrence is now more recognized with the increasing use of MRI and its incidence in pregnancy is estimated to be 11.6 per 100,000 deliveries in the US [48]. Thrombosis of cerebral veins or dural sinuses causes injury to tissue through increased venous pressures and (in the case of dural sinus thrombosis) decreased CSF reabsorption and increased intracranial pressure. The presentation is highly variable and may include headache with or without vomiting, focal deficits, seizures and/or mental status changes. Headache is the most common presentation, with a gradual onset and often localized.
Investigation and management
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree