Fever and host immune response in pregnancy
The pregnant woman may be exposed to any of the common causes of infection. Women in labor and during the puerperium are particularly susceptible to serious infection of the genitourinary tract. In addition, breast and wound infections may occur. Puerperal sepsis (childbed fever) rightly remains the most feared complication of childbirth, still with a significant mortality and morbidity. Box 16.1 summarizes the nonviral causes of fever complicating pregnancy and the puerperium.
The notion that pregnant women become globally immunosuppressed during pregnancy is inconsistent with evolution and the overall favorable survival of the human fetus. Some authors have noted alterations in maternal innate and cellular immunity during pregnancy, as decreased T-cell function and decreased NK-cell function [1–3]. However, scientists have not noted a trend towards either suppression or enhancement of maternal systemic immune function during pregnancy [4].
Fever is a nonspecific indicator of disease, whether infective or noninfective in origin. Fever is also analogous with other nonspecific indicators of an acute inflammatory state such as the erythrocyte sedimentation rate (ESR) or serum C-reactive protein (CRP) level. Irrespective of race or climate, the body temperature normally lies within the range 37.0–37.5°C, with diurnal variation such that evening temperatures are from 0.5–1.0°C higher than the morning level. Oral and axillary temperatures are approximately 0.5°C and 1.0°C lower than the core temperature. Persistent elevation of core temperature above these levels defines fever or pyrexia.
Box 16.1 Nonviral infective causes of fever in pregnancy and the puerperium
Clinical diagnoses
- Endometritis
- Pelvic abscess
- Chorio-amnionitis
- Thrombophlebitis
- Intraperitoneal abscess
- Amniotic fluid embolus
- Mastitis and breast abscess
- Wound infection
- Infective endocarditis
Specific organisms
- Disseminated gonococcal infection
- Syphilis
- Listeriosis
- Tuberculosis
- Severe infection consequent on HIV
Central control of thermal regulation resides in the hypothalamus. Products of tissue injury, however caused, termed endogenous pyrogens, appear to mediate disturbance of thermoregulation. In infective causes of fever, bacterial products such as endotoxin are the cause of cell injury, resulting in the release of endogenous pyrogens from granulocytes, monocytes and fixed macrophages. The resulting fever may be characterized by rises followed by precipitous falls with sweating and peripheral dilation of blood vessels. Increased muscle activity may lead to rigors, resulting in peripheral vasoconstriction and the cold clammy skin that is the hallmark of severe septicemia, particularly with gram-negative organisms.
Fever also has implications for pregnancy in terms of development of the normal fetus. Growing evidence associates intrauterine exposure to hyperthermia and inflammation with adverse neurologic outcomes [5]. In term infants intrapartum fever has been associated with a risk of cerebral palsy, neonatal hypoxic encephalopathy, and seizures. Studies have examined limiting the effects of inflammation and fever on the neonatal brain by giving acetaminophen, steroids and antibiotics, and other neuroprotective agents during planned vaginal deliveries [5,6].
The investigation of fever in pregnancy requires attention not only to the specific infections of pregnancy, but also to the common causes in the community. Therefore, the need for careful history taking cannot be overemphasized. Questions must include symptoms, chronology, recent travel, eating habits, pets and contacts with others with fever. Careful examination of the whole patient is necessary, with particular attention paid to the genitourinary tract, skin rashes, palpable masses and heart murmurs. Investigation should be relevant and thorough, and include genital swabs, urine (with microscopy), and blood cultures (generally two sets drawn from two different sites to help discern the difference between bacteremia and skin contamination). Serum for antibody tests can be obtained to confirm an etiologic diagnosis of specific infections. Two sets of antibodies (IgG and IgM) are usually drawn, “acute” and “convalescent” serum, and are most helpful in confirming a diagnosis when drawn 4–6 weeks apart.
Genital infections in pregnancy
Normal flora and vaginal discharge
Among women of reproductive age, the vaginal flora is a complex ecosystem of aerobic and anaerobic gram-positive and gram-negative bacteria co-existing in close symbiosis. Under the influence of estrogen, the vaginal epithelium contains glycogen, which favors colonization by large gram-positive rods, Lactobacillus spp, which metabolize glycogen to form lactic acid [7]. The resulting pH of less than 4.5 suppresses the growth of potential pathogens, assisted by the production of hydrogen peroxide by lactobacilli. Other bacteria commonly present in large numbers (approximately 107/mL vaginal fluid) include anaerobic and nonbeta-hemolytic streptococci, diphtheroids (Corynebacterium spp) and coagulase-negative staphylococci [8]. Common commensal organisms that are potentially pathogenic include Candida spp, beta-hemolytic streptococci (Lancefield Group B, Streptococcus agalactiae is found in 5–30% of normal vaginas, and less commonly Group A Streptococcus pyogenes), Staphylococcus aureus and Actinomyces spp. Mycoplasmas are also frequent commensals, with Ureaplasma urealyticum present in over 50% of sexually active women [9,10]. Other organisms, for example coliforms, anaerobic gram-negative rods and Gardnerella vaginalis, are often present in low numbers.
Abnormal vaginal discharge is a common problem in both the nonpregnant and pregnant woman. Its particular importance in pregnancy reflects the effect on both outcome and the puerperium. The infective causes of abnormal discharge include viral (herpes simplex), protozoal (Trichomonas vaginalis), fungal (Candida spp) and bacterial infections. Bacterial causes are cervical infection by Neisseria gonorrhoeae and/or Chlamydia trachomatis, and the altered state of microbial flora that characterizes bacterial vaginosis. The characteristics differentiating the common causes of vaginal discharge are shown in Table 16.1.
Table 16.1 Characterization of vaginal discharge
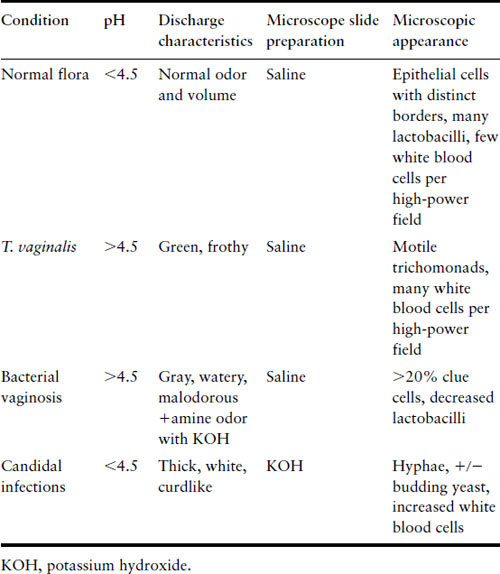
Bacterial vaginosis
Bacterial vaginosis (BV) can be symptomatic or asymptomatic. When symptomatic, it presents as a grayish watery discharge with an odor. Diagnosis can be made clinically using Amsel’s criteria or by gram stain (see Table 16.2) [11]. BV is an alteration of the normal vaginal flora with consequent overgrowth of pathogenic bacteria. G. vaginalis culture or polymerase chain reaction (PCR) is not useful in the diagnosis of BV, given the frequency of G. vaginalis in normal flora. In the past BV has been dismissed as a minor irritation but it is associated with significant adverse pregnancy outcomes. It is a risk factor for premature rupture of membranes with or without labor, preterm labor with intact membranes, chorio-amnionitis, and postpartum endometritis [12–14].
Table 16.2 Criteria for diagnosis of bacterial vaginosis
| Amsel criteria: three out of four of the following criteria should be present: | Gram stain criteria (Nugent score) |
| 0–3: Normal flora |
| 4–7: Intermediate flora |
| 8–10: Bacterial vaginosis |
|
The underlying cause of BV is not understood. The consequence is a fall in the absolute number of hydrogen peroxide-producing lactobacilli, leading to a rise in pH and an increase in the absolute number of G. vaginalis, anaerobic gram-negative rods (Bacteroides spp, Prevotella spp and Porphyromonas spp), Mobiluncus spp and Mycoplasma hominis. The metabolic activity of Mobiluncus spp leads to a release of trimethylamine, producing the foul fishy odor. Exfoliation of vaginal epithelial cells with their adherent G. vaginalis results in the characteristic “clue cells” seen on microscopy of the vaginal discharge [7,8]. An association between pregnancy, BV and human immunodeficiency virus (HIV) infection has also been reported [15].
Box 16.2 Regimens for bacterial vaginosis therapy
- Metronidazole 500 mg orally twice a day for 7 days
- Metronidazole gel, 0.75%, one full applicator (5 g) intravaginally, once a day for 5 days
- Clindamycin cream, 2%, one full applicator (5 g) intravaginally at bedtime for 7 days
Adapted from CDC [34].
There is conflicting evidence about the effect of treatment of BV on outcomes. There are several acceptable treatment regimens. When taking oral metronidazole, patients must avoid alcohol, which produces a disulfiram-like reaction with nausea and vomiting. Current recommendations regarding appropriate treatment for symptomatic bacterial vaginosis in pregnancy are listed in Box 16.2. There are several randomized trials that support the treatment of asymptomatic bacterial vaginosis among women at high risk for preterm birth, particularly when screened early in pregnancy [16–18]. Treatment of asymptomatic bacterial vaginosis in low-risk pregnant women is not currently recommended [19].
Trichomonas vaginalis infection
Trichomonas vaginalis is a sexually transmitted flagellated protozoan. It is the most common pathogenic protozoan of humans in industrialized countries. It causes a purulent vaginitis and can be accompanied by vulvar and cervical lesions, abdominal pain, dyspareunia, and dysuria. It can be diagnosed by wet mount microscopy, which has high specificity but low sensitivity [20–22]. A systematic review of diagnostic tools for T. vaginalis found that PCR and culture have the highest sensitivities and specificities of the various tests available [23].
Trichomonas vaginalis has long been associated with premature rupture of membranes [24,25]. It is also associated with an increased risk of HIV seroconversion in areas of high HIV prevalence [26]. In 1997, the Vaginal Infections and Prematurity Study Group reported a significant increase in low birthweight and preterm delivery [27]. A subsequent prospective randomized trial conducted by the Maternal Fetal Medicine Units Network of the National Institute of Heath has since reported failure of metronidazole to prevent preterm delivery in asymptomatic women infected with T. vaginalis [28]. This trial was stopped early because of an increased risk of preterm birth in the metronidazole group. Of note, the trial used a larger dose of metronidazole than that recommended by the Centers for Disease Control (CDC) for treatment of trichomoniasis in pregnancy. For this reason, screening for asymptomatic trichomoniasis in pregnancy is not recommended. The regimen for treatment of trichomoniasis in pregnancy recommended by the CDC is a single oral dose of 2 g of metronidazole.
Candida infection
Candida is a common vaginal commensal. It is estimated that some 75% of all women will have at least one episode of Candida vaginitis [29]. Asymptomatic carriage in pregnancy occurs in 30–40%, compared with 10–20% of nonpregnant women. Candida vaginitis in pregnancy is not associated with adverse outcome [30]. The vaginal discharge is usually white, curdlike, and odorless and associated with intense itching. The vaginal pH is normal or reduced, in contrast to trichomoniasis or BV. It can generally be diagnosed by wet mount with KOH but certain species are more easily detected with the use of yeast culture. Several species of Candida may be present, but C. albicans predominates (>90%). Treatment is no more difficult than in the nonpregnant woman, but the choice of agent is restricted. Topical nystatin and topical imidazoles (e.g. clotrimazole, econazole, miconazole) for 7 days are safe and effective. The orally active drugs flucytosine and the triazole fluconazole have not been adequately studied in pregnancy.
Neisseria gonorrhoeae infection
The overall prevalence of genital tract infection with N. gonorrhoeae in pregnancy is approximately 1% [31]. The vast majority of these infections are asymptomatic. Infection in pregnancy is associated with premature rupture of membranes and preterm delivery [32,33]. Disseminated gonococcal infection (DGI) is possible in pregnancy and classically presents with oligoarthritis involving the hands, feet and elbows. Fever and skin rash make up the two other parts of the classic triad. The amniotic infection syndrome is a complication of N. gonorrhoeae specific to pregnancy. It is associated with maternal fever, premature rupture of membranes, premature delivery, neonatal infection, and high infant morbidity and mortality. An important consequence of untreated maternal gonococcal infection to the neonate is gonococcal ophthalmia neonatorum. Disseminated infection with arthritis and meningitis occurs, but is rare. Since most infants now receive erythromycin ophthalmic ointment at birth, ophthalmic infection occurs less frequently as well.
Current recommendations include screening by cervical swab for all pregnant women with increased risk (patients under the age of 25, with new or multiple sexual partners, a history of sexually transmitted infections or who are members of a population at increased risk). If using a culture-based diagnostic method, gonococcus does not travel well and a suitable transport medium should be used (e.g. Stuart’s), even for a short transfer. Alternatively, the specimen should be direct plated on to an antibiotic-screened enriched medium (e.g. Thayer Martin), and transported in a candle extinction jar or commercial system to ensure enhanced levels of carbon dioxide in the culture atmosphere. Nucleic acid amplification tests are now becoming the standard diagnostic tool and are widely available. Culture methods should still be used for tests of the pharynx or rectum because of the potential for cross-reactivity with nongonococcal Neisseria from nongenital sites.
The recommended treatment for uncomplicated N. gonorrhoeae in pregnancy is one of the following: a single dose of ceftriaxone 125 mg IM or cefixime 400 mg PO or if unable to tolerate cephalosporins, then spectinomycin 2 g IM [34]. Fluoroquinolones and tetracyclines should be avoided in pregnancy. None of the above regimens is adequate to treat concurrent chlamydial infection, which is likely in women with gonococcal infection (see below).
Chlamydia trachomatis infection
Chlamydia trachomatis is the most common bacterial cause of sexually transmitted infection. The median prevalence among young women attending prenatal clinics is 8%, ranging from 2.8% to 16.9% [35]. Chlamydia trachomatis is also the most common cause of secondary infertility, consequent upon an attack of overt or covert pelvic inflammatory disease (PID). The risk of ectopic pregnancy is increased by 7–10 times following chlamydial PID. As is the case with N. gonorrhoeae, most infected women will be asymptomatic. Risk factors for infection in pregnancy include young age, recent partner change, multiple partners and being unmarried. The routine screening of all pregnant women is recommended by the US CDC, and by the authors and editors. Other bodies have different recommendations. The American College of Obstetricians and Gynecologists (ACOG) recommends testing only in women with a new or more than one sexual partner and those under the age of 25 or with a history of a sexually transmitted infection. The UK National Insitute for Health and Clinical Excellence (NICE) does not recommend routine Chlamydia screening in pregnancy but that women under the age of 25 be given details of their local National Chlamydia Screening Program.
The effect of chlamydial infection on the pregnancy per se is not well understood. The role of chlamydial infection in fetal wastage is not proven. Chlamydia trachomatis has been associated with premature rupture of membranes, prematurity and perinatal death in some studies [36,37] but not in others [38]. A serologic study by Gencay & Koskinieme found an association between raised maternal antichlamydial IgM and increased incidence of chorio-amnionitis, prematurity and perinatal mortality [39].
True congenital infection of the fetus has not been described. However, acquisition of the organism during vaginal birth is common. Up to 70% of babies born to infected mothers will become colonized. In 30–40% this will manifest as conjunctivitis, and in 10–20% as a characteristic pneumonitis. Infection of the vagina, rectum and pharynx may also occur, but may be delayed for up to 7 months, and persist for over 2 years [40]. Chlamydial ophthalmia neonatorum is more common than gonococcal, and is clinically indistinguishable. Up to 50% of babies with gonococcal conjunctivitis will have concurrent chlamydial infection. The common practice of prophylaxis with erythromycin ointment prevents infection in most cases. Chlamydial pneumonitis presents between 3 weeks and 3 months of birth, and is characterized by dyspnea and staccato cough [41].
The diagnosis of chlamydial infection has been revolutionized by the introduction of nucleic acid amplification (NAA) technology, for example PCR and ligase chain reaction (LCR) [42]. Chlamydial NAA can be carried out as a noninvasive test on urine samples from women. It is important that the test manufacturer’s instructions concerning obtaining and transporting specimens are followed closely, and that the testing protocol has been agreed with the diagnostic laboratory.
Because doxycycline is contraindicated in pregnancy, oral azithromycin is the current treatment of choice [43]. The recommended dose is a single oral dose of 1 g azithromycin. Amoxicillin provides alternative therapy in pregnancy, 500 mg three times daily for 7 days. Erythromycin is an alternative drug but is considered to have a decreased efficacy rate, probably related to noncompliance from the gastrointestinal side effects it causes [34].
Mycoplasma infection
Although M. hominis isolated from a case of bartholinitis was the first human Mycoplasma isolate, the role of the genital mycoplasmas in disease is ill defined. As noted above, both M. hominis and U. urealyticum are normal constituents of the genital tract flora. They have been associated with acute chorio-amnionitis, but whether their role is causal remains unclear. There is an apparent association with low birthweight, but this finding is clouded by the association of BV per se with this condition [44–46]. A randomized treatment trial failed to show any improvement in outcome with treatment of U. urealyticum [44].
Mycoplasma hominis can be isolated from blood using conventional blood culture medium, and blood agar incubated micro-aerophilically with 10% CO2. Ureaplasma urealyticum is best isolated using a specific Mycoplasma medium. Based on the evidence that treatment does not improve outcome, there is no justification for routine screening for mycoplasmas during pregnancy.
Mycoplasmas are classically sensitive to tetracyclines, but these drugs should not be used during pregnancy or lactation. Ureaplasma urealyticum is sensitive to erythromycin and other macrolides, but is resistant to clindamycin, whereas M. hominis is resistant to erythromycin and sensitive to clindamycin. This important difference should be borne in mind when a patient with postpartum pyrexia due to presumed endometritis fails to respond to either erythromycin or clindamycin.
Syphilis
Syphilis is a sexually transmitted systemic infection, caused by the spirochete Treponema pallidum. Syphilis has the potential for significant illness in mothers but infection in pregnancy can be disastrous for the fetus. Between 70% and 100% of pregnant women with untreated early syphilis will transmit infection to the fetus, and in up to one-third of cases this will result in stillbirth. The prevalence of primary and secondary syphilis has been climbing since reaching an all-time low in the year 2000. Congenital syphilis, however, continues to decline, likely due to continued prenatal screening and treatment [47]. Maternal symptoms are often mild or nonexistent, and the longer the woman has had untreated syphilis before the first pregnancy, the less likely in utero death will occur, and the more likely a congenitally syphilitic live child will be born. The longer the duration of untreated illness, and the more pregnancies, the less likely that a subsequent fetus will be infected.
The primary lesion is the ulcer or chancer at the site of inoculation, which may be extragenital. The lesion heals in 6–8 weeks and is followed by the secondary phase of systemic spread. Signs include a rash on the palms and feet, patchy alopecia, cervical lymphadenopathy, and flat warty lesions termed condylomata lata. However, symptoms and signs may be transient or absent. The disease then enters the latent phase, divided into early (the first year) and late, when the disease is relatively quiescent. Subsequently, after several years, the cardiologic and neurologic manifestations of tertiary syphilis may develop in up to one-third of untreated patients. See Table 16.3 for the stages and phases of syphilis. Central nervous system involvement can occur in any stage of syphilis. A patient who has clinical evidence of neurologic involvement with known syphilis (e.g. cognitive dysfunction, motor or sensory deficits, ophthalmic or auditory symptoms, cranial nerve palsies, and symptoms or signs of meningitis) should have a cerebrospinal fluid examination.
Table 16.3 Stages and phases of syphilis
| Stage | Phase | Symptoms |
| Primary | Painless chancer | |
| Secondary | Spirochetemia, rash on palms and soles, condyloma lata | |
| Latent | Early <1 year | Asymptomatic |
| Late >1 year | Asymptomatic | |
| Tertiary | Cardiologic and neurologic symptoms in up to 1/3 of untreated people |
Screening for syphilis remains mandatory during pregnancy, because of the importance of identifying infected women early in pregnancy and the availability of effective therapy. Screening is generally carried out either using a nonspecific reagin test such as the rapid plasma reagin test (RPR) or venereal disease research laboratory test (VDRL), or a treponemal specific test such as the Treponema pallidum hemagglutination test (TPHA) or T. pallidum particle agglutination test (TPPA). A positive test with any of these requires confirmation by another unrelated test (reagin or specific), and an adsorbed fluorescent treponemal antigen test (FTA-Abs). The latter test should also be carried out if early syphilis is suspected, for example in the presence of a genital ulcer, because it may become positive early in the disease before the other tests (Table 16.4). A biologic false positive (BFP) can occur with the reagin tests, where other unrelated conditions such as acute viral infection, collagen vascular diseases and even pregnancy itself may give a positive reaction. Screening tests generally remain positive for life, even in patients adequately treated in the past for syphilis or other treponemal infection. If active syphilis cannot be excluded, then the woman must receive an adequate course of therapy.
Table 16.4 Test characteristics of syphilis by stage
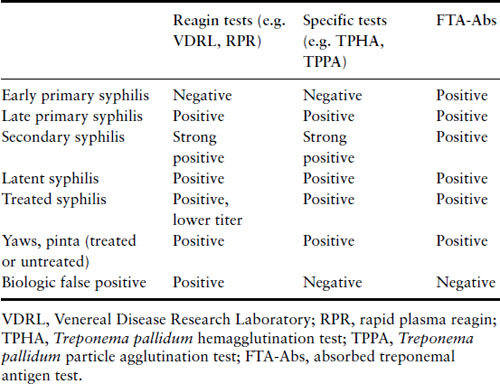
Treatment in pregnancy can successfully decrease the risk of fetal infection. Parenteral penicillin G is the treatment of choice and the regimen should be directed at the maternal stage of disease (Box 16.3) [34]. There is no adequate alternative therapy for women who are allergic to penicillin [48]. Such women should undergo a desensitization protocol and be treated with penicillin [49,50]. This is typically done with administration of sequentially less dilute doses of oral penicillin VK given every 15 minutes and then followed within 30 minutes by a full therapeutic dose by the desired route. It is a potentially life-threatening procedure that requires careful preparation and the ready availability of an experienced resuscitative team.
Box 16.3 Syphilis therapy in pregnancy
| Primary and secondary syphilis | Benzathine penicillin G 2.4 million units IM in a single dose |
| Early latent syphilis | Benzathine penicillin G 2.4 million units IM in a single dose |
| Late latent syphilis | Benzathine penicillin G 7.2 million units total, administered as 3 doses of 2.4 million units IM each at 1-week intervals |
| Tertiary syphilis | Benzathine penicillin G 7.2 million units total, administered as 3 doses of 2.4 million units IM each at 1-week intervals |
| Neurosyphilis | Aqueous crystalline penicillin G 18–24 million units per day, administered as 3–4 million units IV every 4 hours or continuous infusion, for 10–14 days |
Adapted from CDC [34].
As in the nonpregnant state, patients being treated for syphilis should be warned of the possibility of a Jarisch–Herxheimer reaction (an immune reaction to the release of toxins into the body as spirochetal bacteria die), manifesting as an acute febrile response with rigors within 24 hours of starting treatment. Fetal distress and premature labor have been reported [43].
Nongenital infections in pregnancy
Listeriosis
Listeria monocytogenes is a small gram-positive rod, morphologically resembling a diphtheroid. The organism is ubiquitous in nature, with a wide range of temperature tolerance. It is frequently found in soil, and survives and multiplies at 4°C. Contaminated food will not be protected in a domestic refrigerator, and the organism will survive inadequate pasteurization.
There are about 16 serotypes of L. monocytogenes, but only a few are associated with human infection, usually types 4b, 1/2a and 1/2b [51]. The incidence of listeriosis in pregnant women is 12/100,000, 17 times the incidence in the general population [52]. Most patients with perinatal listeriosis are otherwise healthy, but some predisposing conditions include corticosteroid use, diabetes mellitus, autoimmune diseases or HIV infection. Cellular immunity is the primary line of defense against this infection. Animal studies support a role for a depressed cell-mediated immunity increasing sensitivity to listerial infection during pregnancy [53].
Infection in pregnancy is characterized by a biphasic febrile illness. The first stage is usually only considered retrospectively, consisting of nonspecific symptoms such as headache, malaise, backache and abdominal or loin pain, pharyngitis and conjunctivitis – essentially “flu”-like symptoms. The patient may or may not receive antibiotics that by chance are active against L. monocytogenes, and recover. The second attack occurs within 10–15 days of premature delivery, and may reflect reinfection from the contaminated placenta. In about one-third of cases maternal pyrexia in labor will have occurred. Maternal disease can be severe, leading to respiratory distress. However, meningitis (a feature of adult disease) is unusual in pregnancy.
Infants infected during pregnancy are ill at birth or within hours, whereas infants infected at birth will develop late-onset disease 5–7 days later. Predominant features include respiratory distress, bradycardia or apnea, cyanosis, hepatosplenomegaly and jaundice. Neonatal listeriosis can also present with meningitis, as noted in one case series [54]. In one-third of babies a papular rash is found. The cardinal feature of infection is miliary necrosis of the tissues, best seen as white nodules on the cut surface of the placenta or abscesses on the maternal surface [55].
When suspected, listeriosis during pregnancy can be diagnosed by vaginal/cervical swab, stool, urine, culture of amniotic fluid or maternal blood. Maternal blood cultures have been noted to be positive in more than one-third of cases, and are an important part of the diagnostic work-up of a pregnant patient with flu-like symptoms [54,56]. When clinical suspicion is raised, culture for listeriosis should include a tube that is incubated at 4°C in order to maximize the potential for recovery of L. monocytogenes.
Listeria monocytogenes is sensitive to a number of antibiotics in vitro. What is unusual about its sensitivities, however, is that while it is sensitive to penicillins, it is generally resistant to cephalosporins and so it is often not “covered” by many standard antibiotic regimens used to treat infection in obstetrics and gynecology. Optimal therapy is with ampicillin or amoxicillin plus gentamicin depending on severity. Second-line therapy is trimethoprim/sulfamethoxazole (TMP/SMX). If there is a history of penicillin allergy reported, allergy testing or desensitization should be considered. Treatment should be prolonged for 1 week after resolution of fever.
Emphasis is on prevention, and all pregnant women should be counseled on the importance of food hygiene (cook meats thoroughly and store and handle raw meat separately from other foods) and the potential risks of unpasteurized foods (soft cheeses) and ready-to-eat meats, paté and refrigerated smoked seafood.
Toxoplasmosis
Toxoplasma gondii is an obligate intracellular protozoan parasite that is worldwide in distribution. Infection is benign in immunocompetent persons and often individuals are asymptomatic. Some persons may develop a febrile illness with cervical lymphadenopathy. Untreated, the condition is self-limiting as the parasitemia subsides and the organisms become dormant in the tissues, commonly brain, heart and skeletal muscle. They may remain viable for the life of the host, and become reactivated should immunosuppression occur, such as following infection with HIV. This infection also has important implications for the pregnant host. Unfortunate timing may result in primary infection during pregnancy with transmission to the fetus, resulting in serious congenital sequelae.
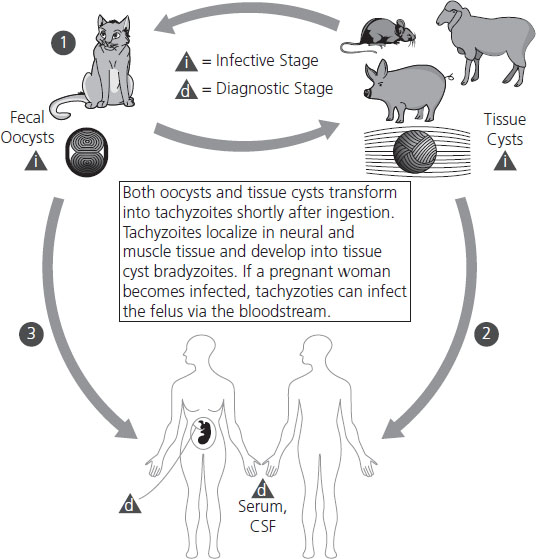
Three life forms of T. gondii occur and are reviewed in Figure 16.1: the oocyst, the tachyzoite, and the bradyzoite. The oocyst is the product of the sexual cycle in the small intestine of cats. Sporulation of the oocyst is required for its infectivity and occurs after its excretion into the environment. Sporulation is more rapid at higher temperatures. The soil becomes an environmental reservoir, for the cysts remain viable for up to 18 months in moist soil. The tachyzoite form is invasive and is seen during acute infection. They can infect nearly all mammalian cells. Bradyzoites are found in tissues as cysts and are slow growing. Most persist in tissues for life. Cysts containing bradyzoites are commonly found in three distinct areas: myocardium, skeletal muscle, and brain. Tissue cysts are resistant to digestive juices, so they transmit infection well in raw or undercooked meat.
Figure 16.1 Life cycle of Toxoplasma gondii. Members of the cat family (Felidae) are the only known definitive hosts for the sexual stages of T. gondii and thus are the main reservoirs of infection. Cats become infected with T. gondii by carnivorism. After tissue cysts or oocysts are ingested by the cat, viable organisms are released and invade epithelial cells of the small intestine where they undergo an asexual followed by a sexual cycle and then form oocysts, which are excreted. The unsporulated oocyst takes 1–5 days after excretion to sporulate (become infective). Although cats shed oocysts for only 1–2 weeks, large numbers may be shed. Oocysts can survive in the environment for several months and are remarkably resistant to disinfectants, freezing, and drying, but are killed by heating to 70°C for 10 minutes. Human infection may be acquired in several ways: (1) ingestion of undercooked infected meat containing Toxoplasma cysts; (2) ingestion of the oocyst from fecally contaminated hands or food; (3) organ transplantation or blood transfusion; (4) transplacental transmission; (5) accidental inoculation of tachyzoites. The parasites form tissue cysts, most commonly in skeletal muscle, myocardium, and brain; these cysts may remain throughout the life of the host. From CDC www.dpd.cdc.gov/dpdx.
The predominant mode of transmission is eating infected, undercooked meat. As many as 25% of lamb and 25% of pork samples have been shown to contain tissue cysts [57]. Cysts have been rarely isolated from beef, eggs, and unpasteurized milk. Maternal transmission occurs in several ways:
- ingestion of oocysts due to not washing hands after handling soil or cat litter
- consumption of oocysts due to contaminated food or water
- ingestion of bradyzoites/tachyzoites from the consumption of meat or meat products
- blood transfusion of tachyzoites
- inhalation of oocysts from dust.
Maternal infection is frequently unnoticed or presents as a self-limiting nonspecific illness. Women may present with nontender lymphadenopathy (involving the bilateral posterior cervical nodes), fatigue, fever, headache, malaise, and myalgia. The clinical scenario may be confused with mononucleosis, influenza or an alternative nonspecific viral illness. Estimates of maternal infection are unreliable, and observed rates are much lower than expected rates. A sample US survey from 1988–1994 showed Toxoplasma IgG seroprevalence to be 15% among women aged 15–44 years [58]. The prevalence of toxoplasmosis infection in pregnant women has previously ranged from 10% in the United Kingdom and Norway to around 55% in France and Greece [59]. Screening programs, although not popular in the US, have been conducted in European countries such as France and Norway. A screening program in Norway examined 35,940 pregnant women with serology and amniotic fluid for PCR. Forty-seven women (0.17%) showed evidence of primary infection during pregnancy [60].
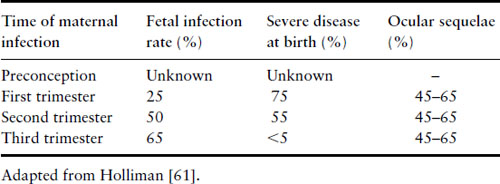
Fetal infection results from placental infection and transmission of parasites following a primary maternal infection accompanied by parasitemia. The effect on the fetus depends on the time of maternal primary infection in relation to gestation. The earlier the infection, the lower the chance of fetal infection, but the more likely severe involvement (Table 16.5) [61]. An estimated 400–4000 cases of congenital toxoplasmosis occur in the US each year [62].
Table 16.5 Transmission of toxoplasmosis in pregnancy
Establishment of infection in the placenta may lead to congenital infection. Severe early infection may lead to abortion or stillbirth. The classic triad of intracranial calcification, hydrocephalus and chorioretinitis is the most extreme form, but is not common. The majority (>90%) of infants are asymptomatic at birth. Manifestations of the disease are numerous, including encephalitis, epilepsy, mental and growth retardation, jaundice, hepatosplenomegaly, thrombocytopenia and skin rashes.
Testing for toxoplasmosis is far from straightforward. Preconception testing establishes those who have previously been infected, and will not therefore have an infected child, or those susceptible who can be counseled concerning contact with cats, uncooked food and general hygiene. The problem surrounds the interpretation of tests performed during pregnancy. The Toxoplasma latex agglutination test should not be used, as it is prone to false-positive as well as false-negative results. In pregnancy, serum should always be sent to a Toxoplasma reference laboratory for determination of specific anti-Toxoplasma IgM. The presence of IgM-positive serum indicates recent infection, but the problem is to determine how recent, in order to guide the clinician and patient regarding the probability of severe congenital infection. A reference laboratory can help narrow down the time of infection with tests such as the IgG avidity test, the Sabin–Feldman dye test, IgM enzyme-linked immunosorbent assay (ELISA), and the differential agglutination (AC/HS) test [63]. The only certain way of positively identifying acute infection is the demonstration of seroconversion. In acute infection, IgG and IgM antibodies generally rise within 1–2 weeks of infection. IgM antibodies have been reported to persist for up to 18 months post infection. A negative IgM with a positive IgG result indicates infection at least 1 year previously. A positive IgM result may indicate more recent infection or may be a false-positive reaction. In 1997 the FDA distributed an advisory to US physicians on how to interpret commercial test results (Table 16.6). Controversy continues over whether antenatal screening should be routine. However, the imprecision of the currently available tests and the relatively low incidence of congenital disease in the US would suggest that the extra uncertainty and anxiety would not justify any possible benefit.
Table 16.6 Guide to general interpretation of T. gondii serology results obtained with commercial assays (FDA 1997)
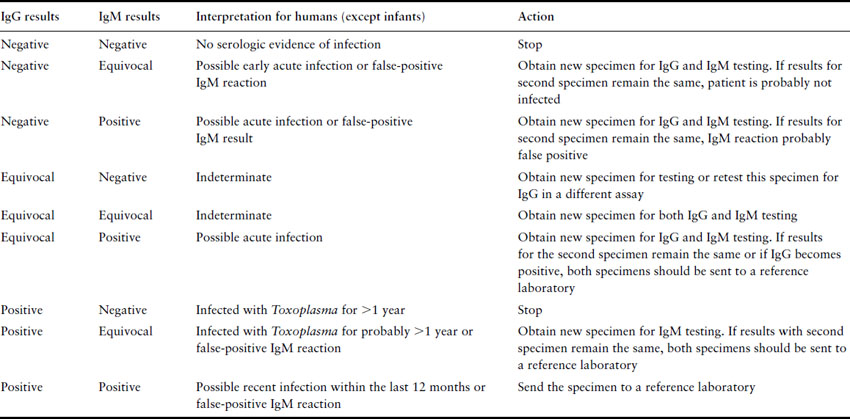
If acute infection of the mother is likely, then ultrasound and amniocentesis should be considered to determine whether congenital infection is established. Amniotic fluid can be sent for PCR testing and has proven to be more sensitive and safer than fetal blood sampling. Ideally, the amniocentesis is performed in the second trimester and at least 4 weeks after acute maternal infection. Although not without risk, the result is important because it may influence the choice of anti-infective drugs and whether to continue with pregnancy. Ultrasound has also been helpful in identifying fetal abnormalities associated with congenital toxoplasmosis. In one study notable findings included ventricular dilation, intracranial densities, increased placental thickness and/or hyperdensity, intrahepatic densities, hepatomegaly, ascites, and pericardial and/or pleural effusion [64]. Ultrasound becomes more sensitive as the pregnancy progresses, and serial ultrasounds are usually suggested.
Mothers thought to be suffering from acute Toxoplasma infection who either do not seek termination or in whom termination is not indicated require antimicrobial therapy for the duration of the pregnancy. The drug of choice is the macrolide spiramycin, which can safely be given throughout pregnancy. Spiramycin concentrates in the placenta and is thought to prevent vertical transmission. To determine if the fetus should be treated, PCR of the amniotic fluid is performed in the second trimester. If the PCR is negative, spiramycin is continued. If the PCR is positive, concern for congenital infection is warranted and the recommendation is to give sulfadiazine and pyrimethamine (or courses of pyrimethamine/sulfadiazine alternating every 3 weeks) until delivery. Side effects of bone marrow and renal toxicity should be monitored when giving sulfadiazine and pyrimethamine. Folinic acid 10–25 mg daily is added to this regimen to prevent bone marrow suppression. It should be noted that although some studies indicate a reduction in congenital infection with these regimens, an individual patient data meta-analysis found “weak evidence for an association between early treatment and reduced risk of congenital toxoplasmosis” [65], and there are no double-blind placebo-controlled trials reported.
Malaria
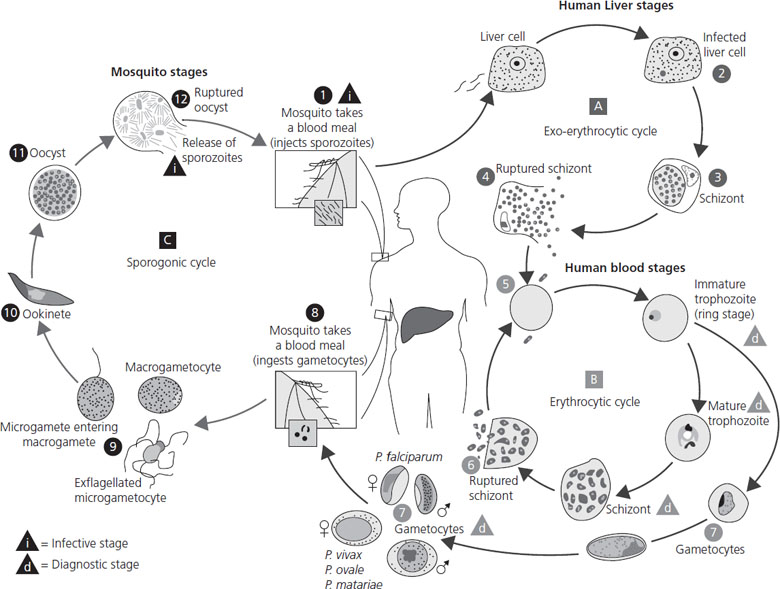
Plasmodium infection should be considered in any patient with fever and/or jaundice who lives in or has recently traveled through Africa, South East Asia, India or South America. Pregnant women have an increased incidence of infection, and when the infection does occur, it is more likely to be severe and associated with a higher level of parasitemia. This may in part be due to immunologic changes in pregnancy. However, there is also evidence that malarial parasites in pregnant women can express a variant surface antigen on infected red blood cells that facilitates binding to chondroitin sulfate A in the placenta [66,67]. The ability for the malarial parasite to infect the placenta contributes directly to the increased morbidity and mortality seen with pregnancy-associated malaria [68,69,70]. The life cycle of Plasmodium species is reviewed in Figure 16.2.
Figure 16.2 Life cycle of malaria. The malaria parasite life cycle involves two hosts. During a blood meal, a malaria-infected female Anopheles mosquito inoculates sporozoites into the human host. Sporozoites infect liver cells and mature into schizonts, which rupture and release merozoites. (Of note, in P. vivax and P. ovale a dormant stage (hypnozoites) can persist in the liver and cause relapses by invading the bloodstream weeks or even years later.) After this initial replication in the liver (exo-erythrocytic schizogony), the parasites undergo asexual multiplication in the erythrocytes (erythrocytic schizogony). Merozoites infect red blood cells. The ring stage trophozoites mature into schizonts, which rupture, releasing merozoites. Some parasites differentiate into sexual erythrocytic stages (gametocytes). Blood stage parasites are responsible for the clinical manifestations of the disease. The gametocytes, male (microgametocytes) and female (macrogametocytes), are ingested by an Anopheles mosquito during a blood meal. The parasites’ multiplication in the mosquito is known as the sporogonic cycle. While in the mosquito’s mid-gut, the microgametes penetrate the macrogametes, generating zygotes. The zygotes in turn become motile and elongated (ookinetes) which invade the mid-gut wall of the mosquito where they develop into oocysts. The oocysts grow, rupture, and release sporozoites, which make their way to the mosquito’s salivary glands. Inoculation of the sporozoites into a new human host perpetuates the malaria life cycle. From CDC www.dpd.cdc.gov/dpdx.
Systemic effects of malaria in pregnancy in patients without prior infection are often pronounced, the most severe including hemolytic anemia, thrombocytopenia, hypoglycemia, respiratory failure and lactic acidosis. Hepatorenal syndrome is often the cause of death. For patients who live in endemic areas and therefore have some partial immunity, the clinical presentation may be much less severe and even asymptomatic. Malaria may infect the placenta, leading to maternal anemia, spontaneous abortion, stillbirth and low birthweight. Malaria parasites may cross the placenta, particularly in nonimmune mothers, leading to congenital malaria. Immune primigravidae are prone to relapse in the second trimester [71,72]. The placenta of patients treated for malaria in pregnancy should always be examined histologically for the presence of parasites. If these are present, the neonate is at risk and should be given a course of antimalarial therapy.
Treatment depends on the local area, the dominant Plasmodium type and pattern of drug resistance. In theory, chloroquine would be the treatment of choice for severe infestation with Plasmodium falciparum in chloroquine-sensitive areas, and its use in pregnancy is supported by most authorities. It is clearly not a major teratogen although there may be some infrequent fetal effects [72–74].
For chloroquine-resistant P. falciparum, a combination of oral quinine sulfate and clindamycin is used. While there are old reports of congenital anomalies in infants who survived high-dose quinine used as an abortifacient, the applicability of this information to its use as an antimalarial agent is doubtful. At the doses used to treat malaria, it is clearly not a major teratogen. In a collaborative perinatal study of 106 women exposed to quinine in early pregnancy, there was no increase in frequency of congenital malformation [75]. It use in pregnancy has the added benefit of preventing premature contractions and fever in late pregnancy but it is not likely to be a tocolytic [76]. However, pregnant patients on quinine should have their glucose monitored as quinine-induced hyperinsulinemia has been reported in up to 50% of pregnant women with severe falciparum
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


