Anterior pituitary
Anterior pituitary hormone changes in pregnancy
During pregnancy, the normal pituitary gland enlarges considerably, due to the estrogen-stimulated lactotroph hyperplasia [1,2], Prolactin (PRL) levels rise gradually throughout gestation, preparing the breast for lactation [3]. Thus, the finding of hyperprolactinemia in a woman with amenorrhea could well be due to pregnancy and not to pathologic hyperprolactinemia. This lactotroph hyperplasia results in an increase in overall pituitary size as seen on magnetic resonance imaging (MRI) scans, with the peak size occurring in the first few days post partum when gland heights up to 12 mm may be seen [4,6]. Following delivery, there is a rapid involution of the hyperplasia, so that normal pituitary size is found by 6 months post partum (5,6). This stimulatory effect of pregnancy on lactotrophs has important implications for the patient with a prolactinoma who desires pregnancy.
Beginning in the second half of pregnancy, circulating levels of a growth hormone (GH) variant made by the syncytiotrophoblastic epithelium of the placenta increase and pituitary GH secretion decreases [7,8]. The decreased production of normal pituitary GH is likely due to negative feedback effects of insulin-like growth factor 1 (IGF-1), which is stimulated by the placentally produced GH variant [7,8]. In patients with acromegaly who have autonomous GH secretion and become pregnant, both forms of GH persist in the blood [9].
Cortisol levels rise progressively over the course of gestation, resulting in a two- to threefold increase by term [10]. Most of this elevation is due to the estrogen-induced increase in cortisol-binding globulin (CBG) levels [11]. However, the cortisol production rate is increased, resulting in a threefold increase in the bio-active “free” fraction, urinary free cortisol levels and salivary cortisol levels [10,11]. Adrenocorticotropic hormone (ACTH) levels have been variously reported as being normal, suppressed or elevated early in gestation [10,12]. However, later in the pregnancy, there is a progressive rise, followed by a final surge of ACTH and cortisol levels during labor [10]. ACTH does not cross the placenta but it is also manufactured by the placenta [12]. The amounts of ACTH in maternal serum that are of placental as compared to pituitary origin at various stages of gestation are not known. Corticotropin-releasing hormone (CRH) is also produced by the placenta and is released into maternal plasma [13]. The CRH is bio-active and may release ACTH both from the placenta, in a paracrine fashion, and from the maternal pituitary [13,14]. The role of placental CRH in regulating ACTH and cortisol secretion during pregnancy in humans is still unclear but it may be driving the marked increase in ACTH and cortisol in the third trimester [14].
Thyroid stimulating hormone (TSH) levels fall in the first trimester, in response to the rise in thyroid hormone levels which are stimulated by human chorionic gonadotropin (HCG), but then return to the normal range by the third trimester [15]. In response to placental sex steroid production, both hypothalamic gonadotropin-releasing hormone (GnRH) and pituitary gonadotropin (follicle-stimulating hormone (FSH)/luteinizing hormone (LH)) levels decline in the first trimester of pregnancy, with a blunted gonadotropin response to GnRH [16].
Pituitary tumors
Pituitary adenomas are quite common in women, comprising nearly 6% of intracranial (malignant and nonmalignant) neoplasms [17]. They may cause problems because of oversecretion of hormones by the tumor as well as by causing hypopituitarism, thereby affecting fertility and pregnancy outcome if pregnancy does ensue. In addition, the pregnancy itself alters hormone secretion and pituitary function, complicating the evaluation of patients with pituitary neoplasms. The influence on various types of therapy on the developing fetus also affects therapeutic decision making.
Prolactinoma
Hyperprolactinemia is responsible for about one-third of all cases of female infertility [18]. Hyperprolactinemia impairs the hypothalamic–pituitary–ovarian axis at several levels, the primary site of inhibition being at the hypothalamus where it inhibits the pulsatile secretion of GnRH [19]. The differential diagnosis of hyperprolactinemia is extensive [19,20] but this discussion will focus on the patient with a prolactinoma.
For patients with prolactinomas, the choice of therapy may have important consequences for decisions regarding pregnancy. Transsphenoidal surgery is curative in 50–60% of cases and rarely causes hypopituitarism when it is performed on women with microadenomas (tumors <10 mm in diameter). For patients with macroadenomas (tumors ≥10 mm in diameter), surgery cures a much smaller number with a considerably greater risk of causing hypopituitarism, and may therefore affect fertility [21].
Dopamine agonists, including bromocriptine, pergolide (approved for the treatment of Parkinson’s disease but not hyperprolactinemia in the US), quinagolide (not approved in the US) and cabergoline, have become the primary mode of therapy for virtually all patients with prolactinomas [20,21]. Bromocriptine, pergolide and quinagolide can restore ovulatory menses in 70–80% of women and cabergoline can do so in over 90% [20–22]. Once ovulatory menses have been established, mechanical contraception is advised until the first two or three cycles have occurred, so that a woman will know when she has missed a menstrual period and the drug can be discontinued after confirmation with a pregnancy test. In this way, these drugs will have been given for only about 3–4 weeks of the gestation. However, because of its long half-life in the body, cabergoline cessation at that point will result in fetal exposure for an additional week or more.
In addition to their efficacy in lowering PRL levels, dopamine agonists often reduce tumor size of PRL-secreting macroadenomas, bromocriptine reducing the size by ≥50% in 50–75% of patients [21,23] and cabergoline achieving such reductions in over 90% of patients in some series [21,24,25].
Effects of pregnancy on prolactinoma growth
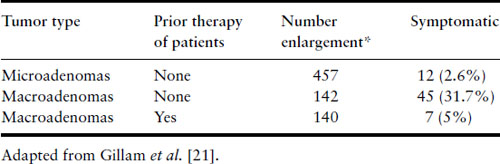
The stimulatory effect of the hormonal milieu of pregnancy may result in significant prolactinoma enlargement during gestation (Figure 13.1). Tumor enlargement may also occur because the dopamine agonist that had caused the tumor to shrink has now been discontinued. Data have been compiled from five studies that analyzed the risk of symptomatic tumor enlargement in pregnant women with prolactinomas, divided according to tumor size [26–30] (Table 13.1). For women with microadenomas, only 12 of 457 pregnancies (2.6%) were complicated by symptoms of tumor enlargement (headaches and/or visual disturbances). Surgical intervention was not required in a single case and medical therapy with reinstitution of bromocriptine resolved the symptoms in the five in whom it was tried. In 45 of 142 pregnancies (31%) in women who have not undergone prior surgery or radiotherapy for their macroprolactinomas, there were similar symptoms of tumor enlargement. Of these 45, surgical intervention was undertaken in 12 and medical therapy in 17, leading to resolution of their symptoms. One hundred and forty women with macroadenomas have been identified who have undergone surgery or radiation prior to pregnancy; their risk of tumor enlargement was low (5%). If tumor enlargement occurs, reinstitution of bromocriptine or cabergoline usually is successful in reducing the size of the tumor, but transsphenoidal surgery may be necessary [20,21,31].
Figure 13.1 Coronal and sagittal MRI scans of an intrasellar prolactin-secreting macroadenoma in a woman prior to conception (above) and at 7 months of gestation (below). Note the marked tumor enlargement at the latter point, at which time the patient was complaining of headaches. Reproduced with permission from Molitch ME. Medical treatment of prolactinomas. Endocrinol Metab Clin North Am 1999;28:143–70.
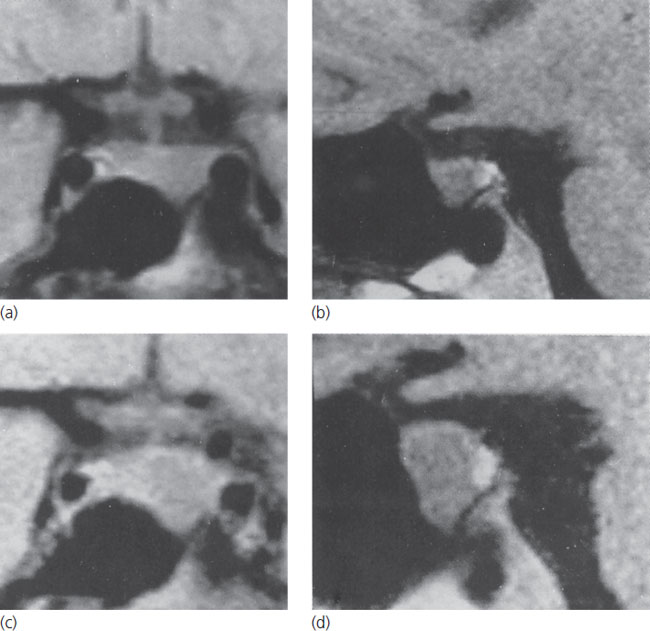
Table 13.1 Effect of pregnancy on prolactinomas
Effects of hyperprolactinemia and its treatment on pregnancy
Bromocriptine crosses the placenta [32]. When taken for only the first few weeks of gestation, it has not been associated with any increase in spontaneous abortions, ectopic pregnancies, trophoblastic disease, multiple pregnancies, congenital malformations or perinatal abnormalities (Table 13.2) [33,34]. Long-term follow-up studies of 64 children between the ages of 6 months and 9 years whose mothers took bromocriptine in this fashion have shown no ill effects [35]. Experience is limited to only just over 100 women, however, with the use of bromocriptine throughout gestation, but no abnormalities were noted in the infants, except one with an undescended testicle and one with a talipes deformity [36]. Pergolide has been shown to cross the placenta in mice [37]and limited data suggest that there is an unacceptable risk of congenital malformations [38,39]. Some early publications reported no detrimental effects on pregnancy or fetal development in women who became pregnant during treatment with quinagolide [40]. However, a review of 176 pregnancies reported 24 spontaneous abortions, one ectopic pregnancy, one stillbirth and nine fetal malformations [41]. Therefore, we do not recommend that pergolide or quinagolide be used if pregnancy is desired. In the US, pergolide has recently been withdrawn from use because of its association with cardiac valvular abnormalities.
Table 13.2 Effect of bromocriptine on pregnancies
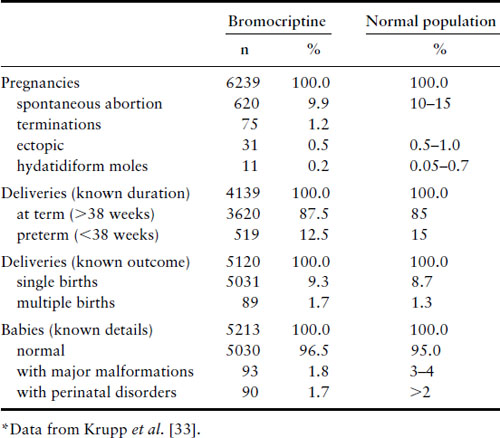
Cabergoline has been shown to cross the placenta in animal studies, but such data are lacking in humans. Data on exposure of the fetus during the first several weeks of pregnancy have been reported in just over 350 cases and such use has not shown an increased percentage of spontaneous abortion, premature delivery, multiple pregnancy or congenital abnormalities [42–46]. Available data from 107 children whose mothers had taken cabergoline in the first few weeks of gestation and who were followed for 1–72 months showed normal physical and mental development [42].
In conclusion, with respect to using a dopamine agonist to facilitate ovulation and fertility, bromocriptine has the largest safety database and has a proven safety record for pregnancy. Although the database for cabergoline use in pregnancy is much smaller, it does not appear that cabergoline exerts any deleterious effects on pregnant women and the incidence of malformation in their offspring is not greater than in the general population. The safety databases for pergolide and quinagolide are quite limited, but they raise concerns and so these drugs should not be used when fertility is desired. The effects of transsphenoidal surgery during gestation are not known specifically, but would not be expected to be significantly different from the effects of other types of surgery [47] unless hypopituitarism should ensue.
Management of prolactinoma in pregnancy
For patients with microadenomas or intrasellar macroadenomas, bromocriptine or cabergoline therapy is generally preferred to surgery because it is safe for the fetus when discontinued early in gestation and poses only a small risk of tumor enlargement for the mother. Such patients should be assessed each trimester for symptoms such as headaches or visual problems; visual field testing need only be done when clinically indicated. When the tumor is large or extends to the optic chiasm or into the cavernous sinus, the following approaches should be considered: (1) pre-pregnancy surgical debulking; (2) frequent monitoring without dopamine agonist therapy unless symptoms develop; or (3) continuous dopamine agonist therapy. The second option is usually chosen by most clinicians. The safety of continuous dopamine agonist therapy during pregnancy has not been established, but based on the small number of cases cited earlier for bromocriptine, it probably is not harmful. Patients with macroadenomas should be assessed monthly for symptoms of tumor enlargement and visual fields should be tested each trimester. PRL levels, which normally increase in pregnancy, may not rise in women with prolactinomas [48]. Conversely, PRL levels may not always rise with pregnancy-induced tumor enlargement [48]; therefore, periodic measurements of PRL levels are of little benefit and may even be misleading.
When there is evidence of tumor enlargement during pregnancy, dopamine agonist therapy should be reinstituted immediately and the dosage increased as rapidly as tolerated. Such therapy must be monitored closely and transsphenoidal surgery or delivery (if the pregnancy is far enough advanced) should be considered if there is no response to the dopamine agonist [20,21].
Although suckling stimulates PRL secretion in normal women for the first several weeks post partum, there are no data to suggest that breastfeeding can cause tumor growth. Thus, there is no reason to discourage nursing in women with prolactinomas but, of course, dopamine agonist treatment must be withheld during breastfeeding.
In some women, postpartum PRL levels are considerably lower than prepartum levels so that menses may resume spontaneously and resumption of dopamine agonist therapy may not be necessary. Therefore, resumption of dopamine agonist therapy post partum should not be routine.
Acromegaly
Reports of pregnancy in patients with acromegaly are uncommon [49–60], perhaps because 30–40% of such patients have hyperprolactinemia [61]. Correction of hyperprolactinemia with a dopamine agonist may be necessary to permit ovulation and conception in these patients [50,56]. Most patients with acromegaly are treated with surgery as primary therapy and those not cured by surgery are usually treated medically [49]. The primary medical therapy is a somatostatin analog and both octreotide LAR and lanreotide autogelare used [49]. Cabergoline may also be helpful, usually as add-on therapy to a somatostatin analog but occasionally as monotherapy [49].
Diagnosis of acromegaly during pregnancy
Conventional immunoassays for GH cannot distinguish between normal pituitary GH and the placental GH variant [7]. Special immunoassays using antibodies that recognize specific epitopes on the two hormones [7] must be used in this regard. When such specific assays are not available, it may be necessary to wait until after delivery to assess pituitary GH secretion accurately, because the placental variant falls to undetectable levels within 24 hours [7]. However, there are two differences between the secretion of the placental GH variant and the secretion of pituitary GH during acromegaly that may allow a distinction to be made during pregnancy. First, pituitary GH secretion in acromegaly is highly pulsatile, with 13–19 pulses per 24 hours [62], whereas secretion of the pregnancy GH variant is nonpulsatile [9]. Second, in acromegaly, about 70% of patients have a GH response to thyrotropin-releasing hormone (no longer available in the US) [63], whereas the placental GH variant does not respond to this hormone [9].
Effects of pregnancy on tumor size and acromegaly
Only three patients with tumors secreting GH have been reported to have enlargement of their tumors with a resultant visual field defect in one during pregnancy [28,60,64]. However, in one of these three the tumor size had been controlled by somatostatin analogs prior to pregnancy, so how much of the tumor enlargement was due to withdrawal of the somatostatin analog versus the pregnancy cannot be ascertained with certainty [60]. In addition, one patient with acromegaly experienced pituitary apoplexy at 33 weeks [59]. Therefore, patients with acromegaly with macroadenomas should be monitored for symptomatic tumor enlargement in a fashion similar to that for patients with PRL-secreting macroadenomas.
Effects of acromegaly on the pregnancy
Carbohydrate intolerance is present in as many as 50% of patients with acromegaly, and overt diabetes is seen in 10–20% [61]. Insulin resistance secondary to the increased levels of GH may increase the risk of gestational diabetes. There is increased salt retention, and hypertension occurs in 25–35% of patients. In addition, cardiac disease is present in about one-third of patients. There may be a specific cardiomyopathy associated with acromegaly, and coronary artery disease may be increased [61]. Thus, the risks for gestational diabetes, hypertension and heart disease are likely increased in women with acromegaly during pregnancy but there are no specific data to document increased frequencies of these complications.
Management of acromegaly and pregnancy
The considerations regarding the use of bromocriptine and cabergoline in women with prolactinomas also apply to those with acromegaly. For most patients, these drugs should not be continued during pregnancy. Data on the use of somatostatin analogs during pregnancy are limited. Only 14 pregnant patients treated with octreotide, octreotide LAR, and lanreotide have been reported; no malformations were found in their children [57]. Octreotide binds to somatostatin receptors in the placenta [65] and crosses the placenta [66] and therefore can affect developing fetal tissues where somatostatin receptors are widespread, especially in the brain. Because of the limited data documenting safety, we recommend that octreotide and other somatostatin analogs be discontinued if pregnancy is considered and that contraception be used when these drugs are administered. Considering the prolonged nature of the course of most patients with acromegaly, interruption of medical therapy for 9–12 months should not have a particularly adverse effect on the long-term outcome. On the other hand, these drugs can control tumor growth and for enlarging tumors, their reintroduction during pregnancy may be warranted versus operating. Both bromocriptine [58] and octreotide [57] have been started during pregnancy because of tumor enlargement, with successful shrinkage of the tumor and no adverse outcome on the baby being noted.
TSH-secreting tumors
Only three cases of pregnancy occurring in women with TSH-secreting tumors have been reported [67–69]. In one of these cases, octreotide, which had been stopped, had to be reinstituted to control tumor size [67] and in a second, octreotide was continued during pregnancy for tumor size control [68]. The most pressing issue with such tumors is the need to control hyperthyroidism during pregnancy and that can usually be done with standard antithyroid drugs [68]. However, with growing macroadenomas, octreotide may be necessary for tumor size control [67,68] and it is possible that it may be necessary to control the hyperthyroidism if thionamides are ineffective.
Clinically nonfunctioning adenomas
Pregnancy would not expected to influence tumor size in patients with clinically nonfunctioning adenomas (CNFA) and only two cases have been reported in which tumor enlargement during pregnancy resulted in a visual field defect [28,70]. It might be expected that if the normal lactotroph hyperplasia were to occur in a patient with a pre-existing CNFA, this hyperplasia could push up the CNFA to cause chiasmal compression or headaches. In the second case of tumor size increase reported, the patient responded rapidly to bromocriptine treatment, probably due to shrinkage of the lactotroph hyperplasia with decompression of the chiasm and probably with little or no direct effect on the tumor itself [70].
Most CNFA are actually gonadotroph adenomas [71]. Two patients have been reported who had gonadotroph adenomas secreting intact FSH with a resultant ovarian hyperstimulation syndrome [72,73]; both became pregnant, one after having the FSH hypersecretion controlled by bromocriptine [72] and the second following surgical removal of the tumor [73].
Hypopituitarism
Hypopituitarism may occur because of tumor compression of the hypothalamus and/or pituitary stalk or from prior neurosurgery. Hormone deficits can be partial or complete and loss of gonadotropin secretion is common. Induction of ovulation may be difficult and a variety of techniques have been used, including administration of HCG and FSH (in the past as human menopausal gonadotropin [74–77], pulsatile GnRH [78–80] and in vitro fertilization [81]. In a report of 30 courses of fertility treatment over a total of 164 cycles in 19 women, Hall et al. found that two women responded to pulsatile GnRH treatment and the remainder were treated with gonadotropins and HCG, with 98 (68%) of cycles being ovulatory and pregnancy achieved in 18 (11%) cycles [82].
Overton et al. reported on 18 pregnancy outcomes occurring in nine women with hypopituitarism who conceived with ovulation induction; two pregnancies were achieved with a GnRH pump and the remainder with gonadotropins and HCG [83]. Of the 14 singletons, there were five (28%) miscarriages, a number thought to be within the expected range; one-half of the livebirths were small for gestational age but there were no congenital malformations [83]. However, of the four sets of twins, two sets miscarried in the first trimester and two sets ended in intrauterine deaths in the second trimester [83]. Ten of the 11 births were by cesarean section, six being emergencies [83]. Post partum, only one woman successfully breastfed [83]. Thus, these authors considered pregnancies in women with hypopituitarism to be high risk [83].
In adult women, the primary hormone replacements to be considered during pregnancy are thyroid and adrenal hormones. Because of the increased thyroxine turnover and increased volume of distribution that occurs during pregnancy, often T4 levels fall and TSH levels rise with a fixed thyroxine dose over the course of gestation [15,84]. The average increase in thyroxine need in these patients is about 0.05 mg/day. Because patients with hypothalamic/pituitary dysfunction may not elevate their TSH levels normally in the face of increased need for thyroxine, it may be reasonable to increase the thyroxine supplementation by 0.025 mg after the first trimester and by an additional 0.025 mg after the second trimester, also following free T4 levels. However, there are no actual data to support this approach.
Because the cortisol production rate is normally increased in pregnancy [10,11,14], the dose of chronic glucocorticoid replacement theoretically ought to be increased during pregnancy. However, this does not seem to be necessary in practice and patients usually can be kept on their standard replacement doses [14]. Hydrocortisone, prednisolone and prednisone can be used. Hydrocortisone is metabolized by the placental enzyme 11-beta-hydroxysteroid dehydrogenase 2, so the fetus is generally protected from any overdose of hydrocortisone; the usual dose is in the range of 12–15 mg/m2 given in two or three divided doses [14]. Additional glucocorticoids are needed for the stress of labor and delivery, with rapid tapering post partum, and a proposed regimen for doing this is discussed in detail in Chapter 47. Prednisolone does not cross the placenta [85]. Prednisone crosses the placenta in only small amounts [85,86] and suppression of neonatal adrenal function in offspring of women taking prednisone during pregnancy is very uncommon [87]. Glucocorticoids may also pass to the neonate in breast milk, but the amounts (0.14% of maternal blood levels) are not sufficient to alter neonatal adrenal function, even with large maternal doses of prednisone [88].
Adult women with hypopituitarism are being treated with GH replacement in increasing numbers [89]. Although GH was used as an adjunct to fertility treatment with gonadotropins for many years, systematic reviews did not show particular benefit [90] and this treatment has been largely abandoned. On the other hand, there was also little harm. There are few data on the use of GH during pregnancy in hypopituitary individuals and in most series GH therapy has been stopped at conception [91]. As the GH variant, which is biologically active, is produced by the placenta in substantial amounts beginning in the second half of pregnancy and can access the maternal circulation (see above), then at most the mother would be GH deficient only in the first half of pregnancy. When Curran et al. analyzed 25 pregnancies that occurred in 16 patients with GH deficiency during which GH therapy was not continued, they found that there was no adverse outcome of omitting GH therapy on either the fetus or mother and concluded that GH replacement therapy during pregnancy is not essential for GH-deficient women [91]. Given the lack of proven benefit of GH treatment during pregnancy and the absence of reassuring safety data, it seems prudent to stop GH treatment during pregnancy.
Sheehan’s syndrome
Sheehan’s syndrome consists of pituitary necrosis secondary to ischemia occurring within hours of delivery [92,93]. It is usually secondary to hypotension and shock from an obstetric hemorrhage. Pituitary enlargement during pregnancy apparently predisposes to the risk for ischemia with occlusive spasm of the arteries to the anterior pituitary and stalk [92,93]. The degree of ischemia and necrosis dictates the subsequent patient course. It rarely occurs with current obstetric practice [94].
Acute necrosis is suspected in the setting of an obstetric hemorrhage where hypotension and tachycardia persist following adequate replacement of blood products (Table 13.3). In addition, the woman fails to lactate and may have hypoglycemia [92,93,95]. Investigation should include obtaining blood samples for ACTH, cortisol, prolactin, and free thyroxine. The ACTH stimulation test would be normal, as the adrenal cortex would not be atrophied. Free thyroxine levels may prove normal initially, as the hormone has a half-life of 7 days, and an additional sample should be sent after 1 week. Prolactin levels are usually low, although they are generally 5–10-fold elevated in the puerperium.
Table 13.3 Symptoms and signs of Sheehan’s syndrome
| Acute form | Chronic form |
| Hypotension | Light-headedness |
| Tachycardia | Fatigue |
| Failure to lactate | Failure to lactate |
| Hypoglycemia | Persistent amenorrhea |
| Extreme fatigue | Decreased body hair |
| Nausea and vomiting | Dry skin |
| Loss of libido | |
| Nausea and vomiting | |
| Cold intolerance |
Treatment with saline and stress doses of corticosteroids should be instituted immediately after drawing the blood tests. If later free thyroxine levels become low, then therapy with levothyroxine is indicated. Additional pituitary testing with subsequent therapy should be delayed until recovery. Diabetes insipidus may also occur secondary to vascular occlusion with atrophy and scarring of the neurohypophysis [96,97].
When milder forms of infarction occur, the diagnosis of Sheehan’s syndrome may be delayed for months or years [93,95]. These women generally have a history of amenorrhea, decreased libido, failure to lactate, breast atrophy, loss of pubic and axillary hair, fatigue, and symptoms of secondary adrenal insufficiency with nausea, vomiting, diarrhea, and abdominal pain (see Table 13.3) [93,95]. Usually growth hormone and gonadotropins are lost before ACTH and TSH with pituitary damage; however, some women retain gonadotropin secretion and may have normal menses and fertility [95]. Although some women may have episodes of transient polydipsia and polyuria, many demonstrate impaired urinary concentrating ability and deficient vasopressin secretion [97]. Computed tomography (CT) or MRI scans generally reveal partial or completely empty sellae [98].
Lymphocytic hypophysitis
Lymphocytic hypophysitis is thought to be autoimmune in nature, and is manifested by infiltration and destruction of the parenchyma of the pituitary and infundibulum by lymphocytes and plasma cells [99–101]. It generally occurs during pregnancy or the postpartum period [99,101]. It is associated with symptoms of hypopituitarism or an enlarging mass lesion with headaches and visual field defects, and is suspected based on its timing and lack of association with an obstetric hemorrhage or prior history of menstrual difficulties or infertility. It is generally associated with mild hyperprolactinemia (<150 ng/mL) and occasionally with diabetes insipidus. On MRI scans, there is usually diffuse enhancement rather than a focal lesion that might indicate a tumor; definitive diagnosis requires a biopsy [99,101]. However, the clinical picture often allows a clinical diagnosis to be made without invasive procedures.
Treatment is generally conservative and involves identification and correction of any pituitary deficits, especially of ACTH secretion which is particularly common in this condition [99,101]. Data indicating that high-dose corticosteroids are of benefit in treating the destructive process are inconclusive. Surgery to debulk but not remove the gland is indicated in the presence of uncontrolled headaches, visual field defects, and progressive enlargement on scan. Spontaneous regression and resumption of partial or normal pituitary function may occur, although most patients progress to chronic panhypopituitarism [99,101–103]. Other autoimmune disorders may also be associated.
The osmostat, the setpoint for plasma osmolality at which arginine vasopressin (AVP) is secreted, is reduced approximately 5–10 mOsm/kg in pregnancy, dropping from about 285 to 275 mOsm/kg. As a result, pregnant women experience thirst and release AVP at lower levels of plasma osmolality than do nonpregnant women [104]. This reset osmostat and altered thirst threshold is possibly due to high levels of HCG [104]. The placenta produces an amino-terminal peptidase, vasopressinase, an enzyme that rapidly inactivates AVP and oxytocin. Vasopressinase levels increase 1000-fold between the fourth and 38th weeks of gestation [105]. AVP consequently has a 4–6-fold increased metabolic clearance rate during gestation [106,107].
The lower osmostat and increased clearance of AVP by vasopressinase in pregnancy alter the nomograms of plasma osmolality and AVP used in the nonpregnant patient. Serum sodium levels may also be lower than those normally expected in patients with diabetes insipidus [107]. Standard water deprivation tests which require 5% weight loss should be avoided during pregnancy as they may cause uterine irritability and alter placental perfusion. Instead, desmopressin (dDAVP) is used to assess urinary concentrating ability over 11 hours, with a value greater than 700 mOsm/kg considered normal [108]. Urinary concentrating ability in the pregnant patient should be determined in the seated position, as the lateral recumbent position inhibits maximal urinary concentration [104,107]. Delivery of the placenta generally results in a return to normal AVP metabolism in 2–3 weeks.
Plasma oxytocin levels increase progressively during pregnancy, with a dramatic increase at term [109,110]. Oxytocin levels rise further during labor and peak in the second stage. Uterine sensitivity to oxytocin increases with a rise in oxytocin receptors in the myometrium. Hypophysectomy does not alter onset of labor, indicating that oxytocin only facilitates labor [111]. Oxytocin levels rise rapidly during suckling [112].
Diabetes insipidus
Three types of diabetes insipidus may occur in pregnancy: central, nephrogenic or transient vasopressin resistant. Polydipsia, polyuria, and dehydration may occur with all three.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


