Introduction
Pulmonary thromboembolism (PTE) remains a major cause of direct maternal mortality. The UK Confidential Enquiries into Maternal Deaths have highlighted failures in providing appropriate prophylaxis for women at risk, in obtaining objective diagnoses, and employing adequate treatment [1]. Thus the management of these patients is important for the obstetrician, medical consultant and anesthesiologist. Venous thromboembolism (VTE) is up to 10 times more common in pregnant women (defined to include the puerperium and 6–8 weeks post partum) than in nonpregnant women of the same age and complicates about 1/1000 pregnancies [2–4]. Around 85% of these gestational VTE are deep venous thrombosis (DVT) rather than PTE [4–6], as compared to 70% DVT in nonpregnant patients with VTE [7].
The majority of these DVT occur antenatally (65.5%), with distribution across the three trimesters of pregnancy [8,9]. Indeed, almost half of antenatal VTE occurs before 15 weeks gestation [5], emphasizing the need for risk assessment pre-pregnancy and prophylaxis in early pregnancy. However, the rate of VTE is greatest in the puerperium, being almost fourfold that during pregnancy [4–6,8]. Interestingly, almost 90% of pregnancy-associated DVT occur on the left side in contrast to the non-pregnant situation, where only 55% of DVT occur on the left. This may reflect some compression of the left common iliac vein by the right iliac artery. Over 70% of gestational DVT are ileofemoral in their location, which contrasts with around a 9% rate of ileofemoral DVT in the nonpregnant, in whom calf vein DVT predominate [10]. This is important as ileofemoral DVT are more likely to embolize than calf vein thromboses. In the most recent report of the UK Confidential Enquiries into Maternal Deaths, the mortality rate for PTE, the leading cause of direct maternal death, was 1.6 per 100,000 maternities [1]. In the United States, the CDC Pregnancy Mortality Surveillance System (PMSS) has reported rates of fatal VTE during pregnancy of 1.8 and 2.3 per 100,000 livebirths for the periods of 1987–1990 and 1991–1999 respectively [11,12] and PTE was the most frequent cause of maternal death.
Previous VTE is associated with an increased risk of future VTE. The risk of a recurrence of an idiopathic deep venous thrombosis in the general medical population is 10% per year for the first 2 years and 3% per year thereafter. There is also a risk of deep venous insufficiency known as “post-thrombotic syndrome” [13]. This is characterized by chronic persistent leg swelling, pain, a feeling of heaviness, dependent cyanosis, telangiectasia, chronic pigmentation, eczema, associated varicose veins and in some cases lipodermatosclerosis and chronic ulceration. Symptoms are worsened by standing or walking and improve with rest and recumbency. The syndrome is more common where there is a recurrent DVT, with obesity, and where there has been inadequate anticoagulation. Up to 80% of women with VTE develop post-thrombotic syndrome and over 60% will develop objectively confirmed deep venous insufficiency following a treated DVT [14]. The risk of developing venous insufficiency after DVT is greater than with PTE (odds ratio 10.9, 95% confidence interval (CI) 4.2–28.0) for DVT (compared to 3.8 (95% CI 1.2–12.3)) after PTE [14]. This may be due to the thrombus clearing from the leg veins in those with PTE, leading to less extensive venous damage. Berqvist et al. reported that up to 21% of women with a treated DVT in pregnancy required a compression bandage and 6% had venous ulcers at a median follow-up of 10 years [15]. Historical data show rates for venous ulceration following untreated DVT to be 19–28% on follow-up periods ranging from 6 to 31 years [15].
Investigation for venous thromboembolism in pregnancy
Clinical diagnosis of VTE is unreliable, especially in pregnancy. Presenting features (reviewed in Table 3.1) such as dyspnea, cough, chest pain, leg swelling and presyncope/syncope are common in pregnancy. Many young patients with pulmonary embolism may not manifest the typical features of tachypnea, tachycardia, hypotension or hypoxia. Thus any woman with signs and symptoms suggestive of VTE without obvious explanation should have an objective diagnostic investigation performed. Where the likelihood is considered high or there is any delay in such investigation, treatment with low molecular weight heparin (LMWH) should be given until the diagnosis is excluded, unless LMWH treatment is strongly contraindicated.
Table 3.1 Presenting features of pulmonary embolism in nonpregnant patients
Reproduced with permission from Stein et al. [90].
| Symptom/sign | Frequency |
| Shortness of breath | 73% |
| Tachypnea | 70% |
| Pleuritic type chest pain | 66% |
| Crackles on chest auscultation | 51% |
| Cough | 37% |
| Tachycardia | 30% |
| Hemoptysis | 13% |
| Cardiovascular collapse | 8% |
Compression duplex ultrasound is the primary diagnostic test for DVT [16]. If the diagnosis is confirmed, therapeutic LMWH should be continued. In the event that the ultrasound venogram is negative and there is a reasonable alternative explanation for the patient’s symptoms, the investigations may be complete. However, if a high level of clinical suspicion exists, the ultrasound should be repeated in 1 week or an alternative diagnostic test (such as magnetic resonance imagining (MRI) venography, computed tomography (CT) of the iliac veins, pulsed Doppler studies or even conventional venography) employed [16]. If the clinicial suspicion is high enough in some circumstances it may be advisable to continue anticoagulation until this second investigation is completed. If on repeat testing there is no evidence of DVT, treatment can be discontinued [17].
Where PTE is suspected, anticoagulant treatment should be continued until PTE is objectively excluded unless bleeding is considered to be a likely alternative diagnosis. A chest X-ray (CXR) and a duplex ultrasound examination should be conducted. The CXR may identify other pulmonary disease such as pneumonia or pneumothorax [18]. CXR is normal in over half of pregnant patients with objectively proven PTE, but abnormalities suggestive of pulmonary embolism such as atelectasis, pleural effusion, focal opacities, and regional oligemia may be seen [19]. The radiation dose to the fetus from a CXR performed at any stage of pregnancy is negligible [20]. If the CXR does not provide a definitive diagnosis (and it usually will not), bilateral lower limb duplex ultrasound or definitive testing for pulmonary embolism with a CT pulmonary angiogram (CTPA) or ventilation perfusion scan (V/Q) should be carried out. Doppler ultrasound of the legs for the investigation of PTE has not been validated in pregnancy, but several studies in nonpregnant patients with suspected PTE have shown its value [21–23]. The diagnosis of DVT will indirectly confirm a likely PTE. Anticoagulant treatment is the same for both conditions and further investigation may not be necessary, so reducing the number of diagnostic tests and radiation exposure (see below). If the leg compression ultrasounds have not confirmed the presence of thromboembolic disease, or a compression ultrasound is not readily available, then a CT angiogram or ventilation perfusion scan should be obtained.
Currently the decision about whether to use a CTPA or V/Q scan as the primary investigation for PTE will often be determined by local availability and practice patterns. Both are acceptable options with their own advantages and disadvantages. Their relative merits are reviewed in Table 3.2 and discussed below.
Table 3.2 Relative merits of V/Q scan and computed tomography pulmonary angiogram (CTPA) in the investigation of pulmonary thromboembolism (PTE)
Reproduced with permission from Bourjeily et al. [93].
| Parameter | V/Q scan | CTPA |
| Radiation exposure | Minimal radiation to fetus (0.064–0.08 rads). Ventilation scan may be withheld in patients with a normal perfusion scan, further decreasing radiation exposure. Minimal radiation (1.4 mSv) to maternal breast | Minimal radiation to fetus (0.003–0.0131 rads [91]). Significant radiation to maternal breast (2.2–6.0 mSv) that increases risk of breast cancer by 13%. Risk can be decreased by the use of breast shields |
| Ability to offer alternative diagnosis | No | Yes, CTPA diagnoses can identify pulmonary edema, pneumonia and some cases of aortic dissection |
| Ability to offer definitive diagnosis | High probability scan fairly definitive for presence of pulmonary embolism. Low and intermediate probability scan may or may not represent pulmonary embolism with interpretation varying with underlying likelihood of PTE. Normal scan has excellent negative predictive value for PTE [16]. Pregnant patients more likely than general population to have definitive studies. V/Q scan unlikely to be normal in the setting of an abnormal chest X-ray | CTPA likely has better sensitivity and specificity for moderate to large PTE if study is of high quality. Patient movement or variations in timing of intravenous contrast or type of scanner may lead to difficult to interpret studies, especially for smaller peripheral PTE. “Multidetector” CT more likely to identify small PTE than a single-detector CT |
| Need for patient cooperation | Requires considerable time to perform and patient cooperation with positioning and breathing | Relatively brief study that only requires that patient be able to hold breath briefly and lie still on table |
| Invasiveness | Requires intravenous injection and (if perfusion scan abnormal) inhalation of radionuclide | Requires intravenous injection of contrast |
| Ease of access | Varies by institution- handling of radionuclide requires special technician who may not be in hospital after hours. | Varies by institution |
| Ease of interpretation | Interpretation fairly standardized | Interobserver variation in interpretation is considerable. Reading best done by experienced personnel [92] |
Computed tomography pulmonary angiogram is increasingly recommended as the first-line investigation for nonmassive PTE in nonpregnant patients. CTPA has several advantages over V/Q scan, including better sensitivity and specificity, and a lower radiation dose to the fetus. In addition, it can identify alternative diagnoses which may present similarly to PTE such as pulmonary edema, pneumonia or aortic dissection. However, CTPA may not identify small peripheral PTE although modern multidetector-row spiral CT systems now offer increasingly accurate detection of central and peripheral emboli. Despite these potential advantages of CTPA, many authorities continue to recommend V/Q scanning as the first-line investigation in pregnancy because of the low prevalence of chronic chest disease in young women and its high negative predictive value and also its substantially lower radiation dose to pregnant breast tissue.
The average fetal radiation dose with CTPA is less than 10% of that with V/Q scanning during all trimesters of pregnancy [24–26] with estimates that the risk of this translating into a fatal cancer to the age of 15 years in the child is <1/1,000,000 after in utero exposure to CTPA and 1/280,000 following a perfusion lung scan. Thus in the context of a possible PTE which may have fatal consequences, the risk to the fetus from such investigations should not prevent these investigations being conducted.
The main disadvantage of CTPA is the significant radiation dose (of the order of 20 mGy) to the maternal breasts, which is associated with an increased lifetime risk of developing breast cancer. This is particularly relevant, as around 90–95% of such investigations will have a negative result in pregnancy. The delivery of 10 mGy of radiation to a woman’s breast is estimated to increase her lifetime risk of developing breast cancer, possibly by as much as 13.6% [27], but more recently it has been suggested that this is an overestimate [28] and breast shields may be used with CTPA to limit this exposure further. Nevertheless, breast tissue may be especially sensitive to radiation exposure during pregnancy. Thus, many still consider V/Q scans to be the investigation of first choice for young women with a normal CXR, especially if there is a family history of breast cancer or the patient has had a previous chest CT scan [24].
What is our practice? We support using whichever test is readily available at the hospital where the patient is receiving care. When both studies are readily available, we obtain a V/Q scan if the CXR is normal because of the lower dose of radiation to the breast and its strong negative predictive value. If the CXR is abnormal (making a normal V/Q scan highly unlikely), if the patient is unstable or if the patient is in labor, our preference is to obtain a CTPA.
While pulmonary angiography remains the gold standard for diagnosing pulmonary embolism, the test is increasingly less commonly done as confidence in CTPA increases. This is partly because pulmonary angiography is a much more invasive test and partly because, although the radiation dose is still well below the acceptable limits in pregnancy, it does involve the highest radiation exposure to mother and fetus of all the available testing modalities.
D-dimer is a degradation product of cross-linked fibrin that is elevated in up to 75% of hospitalized medical patients due to a wide variety of conditions. The role outside pregnancy of the two most commonly available D-dimer assays is reviewed in Table 3.3. D-dimer, however, is of very limited value in pregnancy. It is increased by the physiologic changes in the coagulation system in pregnancy and so levels become “abnormal” by term and in the postnatal period in most pregnant women, with all of 23 women tested in the third trimester in one study exhibiting elevated D-dimer levels [29]. Furthermore, D-dimer levels can increase if there is a concomitant problem such as threatened miscarriage or pre-eclampsia [30]. Thus a “positive” D-dimer test in pregnancy is not necessarily consistent with VTE and objective testing remains necessary. A low level of D-dimer in pregnancy is compatible, as in the nonpregnant patient, with no VTE being present. Even then, the D-dimer test is not completely reliable; in the nonpregnant patient with a high pretest probability and a highly sensitive D-dimer assay, 4% of DVT will not be identified by the ELISA D-dimer test, increasing to 17% with a moderately sensitive latex agglutination D-dimer assay. Although some have advocated a role for a negative D-dimer test in the investigation of VTE in pregnancy [31], we believe that D-dimer is of no value [32] in this population. In the presence of clinical suspicion of such an important diagnosis, it does not avoid objective diagnostic testing in pregnancy where the patient will usually have an increased pretest probability for VTE in view of the pregnancy-associated risk factors.
Table 3.3 D-dimer testing in nonpregnant patients
| D-dimer test | Characteristics |
| Semi-quantative latex agglutination assay | Rapid but far less accurate than the ELISA test. Can be used to rule out PTE in nonpregnant patients with a low probability of PTE only |
| Quantitative ELISA | More time intensive but increased sensitivity and specificity. Can be used to rule out PTE in nonpregnant patients except for those with a high probability of PTE. |
ELISA, enzyme-linked immunosorbent assay; PTE, pulmonary thromboembolism. Reproduced with permission from Righini et al. [94].
When considering starting anticoagulation, a baseline complete blood count (hemoglobin and platelet count), prothrombin time (international normalized ratio – INR) and activated partial thromboplastin time (APTT) should be obtained. As anticoagulant therapy can be influenced by renal and hepatic function, a creatinine and aspartate aminotransferase (AST) should also be obtained. Performing a thrombophilia screen prior to therapy is a controversial area and therefore is not routinely recommended. The most commonly ordered thrombophilia tests are reviewed in Table 3.4 along with the effects of acute VTE and heparinization on the results. Whether obtaining a thrombophilia screen acutely is warranted in all patients with PTE is controversial because the results of a thrombophilia screen will not influence immediate management of acute VTE. However, it can provide information that can influence the duration and intensity of anticoagulation, such as when antithrombin deficiency or antiphospholipid syndrome is identified.
Table 3.4 Inherited and acquired tendencies to thrombosis and how to test for them in pregnancy
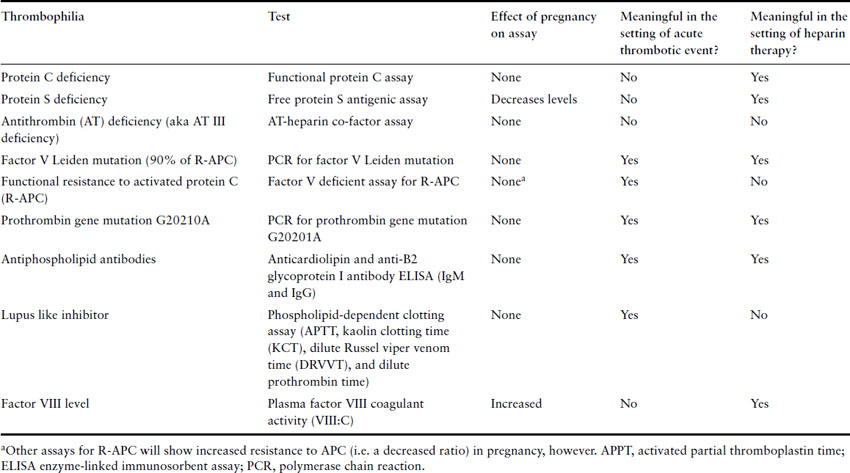
It is important to be aware of the effects of pregnancy and thrombus on the results of a thrombophilia screen. For example, protein S levels fall in normal pregnancy, making it difficult to make a diagnosis of protein S deficiency during pregnancy. Acquired activated protein C resistance is found with the APC sensitivity ratio (a functional assay sometimes used to identify the probability of factor V Leiden gene polymorphism) test in around 40% of pregnancies, linked to the physiologic changes of pregnancy on the coagulation system. Antithrombin may be reduced when extensive thrombus is present. In nephrotic syndrome and pre-eclampsia (conditions associated with an increased risk of thrombosis), antithrombin levels are reduced and in liver disease protein C and S will be reduced. It is important, therefore, that thrombophilia screens are interpreted by clinicians with specific expertise in the area.
Acute treatment of venous thromboembolism
The treatment of VTE in pregnancy is heparin, either in the form of unfractionated heparin (UFH) or LMWH. Potential complications of heparin therapy are reviewed in Table 3.5. The most dangerous of these complications is heparin-induced thrombocytopenia (HIT) and is reviewed in Table 3.6. Meta-analyses of randomized controlled trials (RCT) indicate that LMWH are more effective, are associated with a lower risk of hemorrhagic complications and are associated with lower mortality than UFH in the initial treatment of DVT in nonpregnant patients, and also that LMWH is of equivalent efficacy to UFH in the initial treatment of PTE [33,34]. A large systematic review has concluded that LMWH is a safe alternative to UFH as an anticoagulant during pregnancy [35]. This review found a risk of recurrent VTE of 1.15% when treatment doses of LMWH were used to manage VTE in pregnancy. This compares favorably with recurrence rates of 5–8% reported in trials carried out in nonpregnant patients treated with LMWH or UFH followed by coumarin therapy who are followed up for 3–6 months [36,37] and confirms that LMWH are effective in the treatment of acute VTE in pregnancy. Further, there is evidence that LMWH do not cross the placenta and appear to pose no direct risk to the fetus [38]. LMWH are not associated with an increased risk of severe bleeding peripartum and the risk of HIT is substantially lower compared with UFH [35]. Indeed, in women who have not been exposed to UFH and who are treated with LMWH, some experts believe it may not be necessary to monitor the platelet count (although a baseline platelet count before starting is still advisable) [38,39]. Compared with UFH, LMWH also has a much reduced risk of heparin-induced osteoporosis [35,40].
Table 3.5 Complications of heparin therapy and their likelihood in unfractionated heparin (UFH) versus low molecular weight heparin (LMWH)
| Risk | Comments | Relative frequency with UFH versus LMWH |
| Bleeding | Can occur in up to 5.5% of medical patients on heparin | LMWH less likely to be associated with major hemorrhage [95] |
| Heparin-induced thrombocytopenia (HIT) | Well-recognized complication usually occurring 5–10 days after initiating therapy | HIT is rare in pregnant patients on LMWH |
| Skin necrosis | Rare. Typically occurs in fat-rich areas. Begins with erythema followed by bruising, and then necrosis. | Not known |
| Osteoporosis | Reported in patients on heparin for more than 6 months. Can cause pathologic fractures (in one report in 2.2% of recipients) [96]. Generally felt to be reversible after stopping medication | LMWH causes less osteoclast activation and appears to be much less likely to cause osteoporosis [97] |
| Heparin contamination | Contamination of UFH with oversulfated chondroitin sulfate led to many deaths in 2007. Assays are now used by manufacturers which screen for this contaminant | Contamination has also occurred with some LMWH products |
Table 3.6 Heparin-induced thrombocytopenia (HIT)
Reproduced with permission from Hassell [98].
| Type of HIT | Characteristics |
| Type 1 | Usually occurs in the first 2 days of heparin therapy Nonimmune mechanism likely related to platelet activation by heparin Drop in platelet counts is usually small and resolves when heparin is stopped Not clinically significant except as part of the differential diagnosis of type 2 HIT |
| Type 2 | Usually occurs in the first 5–10 days of heparin therapy Occurs in 0.2–5% of nonpregnant patients on heparin for more than 4 days and is more common in surgical than medical patients Immune-mediated disorder characterized by formation of antibodies againt the heparin-platelet factor 4 complex (platelet factor 4 complex is a heparin-neutralizing protein released from platelet granules when platelets are activated) that leads to widespread platelet activation, aggregation, destruction and often subsequent venous or less commonly arterial thrombosis (HITT) Platelet counts typically drop no more than 60,000/uL and almost never <20,000/uL Diagnosis is largely a clinical one but supported by the 14C-serotonin release assay (which has a 95% sensitivity and specificity), heparin-induced platelet aggregation studies and/or solid phase immunoassays Treatment involves immediate withdrawal of heparin and use of alternative anticoagulants (reviewed in Table 3.7) |
In nonpregnant patients, the recommended therapeutic dose of LMWH varies according to the manufacturer (enoxaparin 1.5 mg/kg once daily, dalteparin 10,000–18,000 units once daily depending on body weight, tinzaparin 175 units/kg once daily). In view of recognized alterations in the pharmacokinetics of dalteparin and enoxaparin during pregnancy, a twice-daily dosage regimen (enoxaparin 1 mg/kg twice daily, dalteparin 100 units/kg twice daily) is usually recommended for these LMWH in the treatment of VTE in pregnancy [17,38]. There are insufficient data to determine if a once-daily dose is adequate and once-daily dosing therefore remains controversial [41]. Preliminary biochemical data from a relatively small number of patients suggest that once-daily administration of tinzaparin (175 units/kg) may be appropriate in terms of anti-Xa acivity in the treatment of VTE in pregnancy [42].
If the diagnosis of VTE is confirmed, treatment with LMWH should be continued. The APTT is not meaningfully changed in patients on LMWH and cannot be used to guide therapy as is done with UFH therapy. Monitoring anti-FXa levels, although still done as an alternative to APTT for patients on LMWH by some groups, is no longer recommended [17] due to the satisfactory results obtained with weight-based dosing and also because anti-Xa monitoring does not predict either recurrent thrombosis or bleeding risk well, at least in part because of variability in the assay [41]. There may be a case for monitoring levels at extremes of body weight (<50 kg and ≥90 kg), and women with other complicating factors including renal disease and recurrent VTE. As noted above, routine platelet count monitoring for evidence of HIT is likely not required in obstetric patients who have received only LMWH. However, if the obstetric patient is receiving LMWH after first receiving UFH, or if she has received UFH in the past, making HIT more likely, the platelet count should be monitored every 2 days from day 4 to day 14, or until LMWH is stopped, whichever occurs first [39,43]. Since UFH is used for a wide variety of medical purposes in which the patient may not always be aware that she has received it, it is the practice of the editors, but not the authors, to monitor platelets in all patients in whom therapeutic LMWH has been initiated.
Intravenous UFH was the traditional method of heparin administration in acute VTE and remains the preferred treatment in massive PTE because of its rapid effect and extensive experience of its use in this situation. In contrast to LMWH, UFH must be monitored and the therapeutic target for the APTT ratio is usually 1.5–2.5 times the average laboratory control value. It should be administered by a weight-based standardized protocol, beginning with a loading bolus of 80 units/kg/h followed by a maintenance infusion of 18 units/kg/h. The first APTT should be checked 4–6 hours after the loading dose, 6 hours after any dose change and then at least daily when in the therapeutic range. However, APTT monitoring of UFH is technically problematic, particularly in late pregnancy when an apparent heparin resistance occurs due to increased nonspecific plasma binding and increased factor VIII and fibrinogen which influence the APTT [17,38]. Therefore, if UFH dose adjustments during the third trimester are based upon a nonpregnant APTT therapeutic range, systematic overdosing of pregnant women could result, possibly increasing the risk of bleeding and osteoporosis. The use of anti-factor Xa heparin assays (target range 0.35–0.67 U/mL, equivalent to heparin level of 0.2–0.4 U/mL by protamine titration) to monitor UFH results in less dose escalation than monitoring with APTT [44]. A variety of nomograms exist for dose adjustment of UFH; however, given that APTT reagents and coagulometers vary markedly in their sensitivity to UFH, institution-specific nomograms should probably be utilized. When patients are converted from intravenous to subcutaneous UFH, a typical approach is to calculate the total 24-hour UFH dose, divide it by two (or three) and administer the dose obtained from this calculation every 12 (or 8 hours). Subsequent adjustments can be based on either the APTT 6 hours after injection or the anti-factor Xa level 2–4 hours after injection. HIT is a real possibility with UFH and platelet count in these patients must be monitored at baseline and every 2–3 days for the first 14 days. Thereafter, we monitor them monthly but HIT becomes very unlikely after the initial 2 weeks. The features of HIT and alternative anticoagulants to use if HIT develops are reviewed in Tables 3.6 and 3.7 respectively.
Table 3.7 Newer anticoagulants and their use in pregnancy
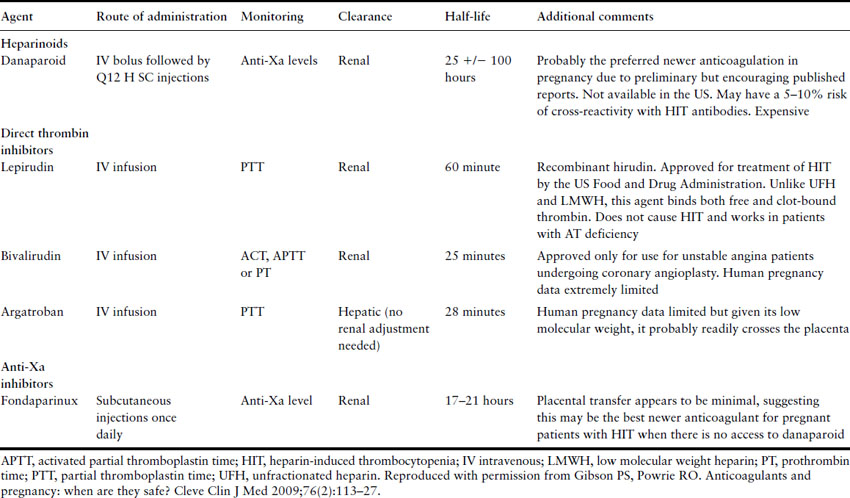
In massive life-threatening PTE with hemodynamic compromise, there is a case for considering thrombolytic therapy as anticoagulant therapy will not reduce the obstruction of the pulmonary circulation. RCT using thrombolytic agents for PTE in nonpregnant patients have established that thrombolysis is more effective than heparin in reducing clot burden and rapidly improving hemodynamics but have not shown any impact on long-term survival [45]. A systematic review of thrombolytic therapy in pregnancy reported on 172 women: 164 treated with streptokinase, three with urokinase and five with rt-PA. There were five nonfatal maternal bleeding complications (2.9%) and three fetal deaths (1.7%) [46]. Overall, data suggest that the maternal bleeding complication rate is in the range of 1–6%, which is consistent with that in nonpregnant patients receiving thrombolytic therapy. It is not known whether these agents cross the placenta but their high molecular weight makes this unlikely. Most bleeding events occur around catheter and puncture sites and, in pregnant women, there have been no reports of intracranial bleeding. If thrombolytic therapy has been given, an infusion of UFH is then started but the loading dose should be omitted.
Pain and swelling in the affected leg are debilitating symptoms of DVT. In patients with proximal DVT, pain and swelling improve faster in mobile patients wearing compression hosiery than in those resting in bed without any compression. Studies in nonpregnant persons have shown that early mobilization, with compression therapy, does not increase the likelihood of developing PTE. Thus there is no requirement for bed rest in a stable patient on anticoagulant treatment with acute DVT [47,48]. Where DVT threatens leg viability through venous gangrene, the leg should be elevated, anticoagulation given and consideration given to surgical embolectomy or thrombolytic therapy.
Maintenance treatment of venous thromboembolism
Antepartum
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


