Thoracentesis
Gregg A. DiGiulio
Introduction
A pleural effusion is a collection of fluid in the potential space that exists between the visceral and parietal pleurae of the lung. The causes of an effusion are varied. In the pediatric patient, acute pulmonary infection is the most common etiology, whereas some other less common etiologies include collagen-vascular disease, congestive heart failure, hypoalbuminemic states, trauma, and neoplasm (1,2). Table 81.1 lists some of the various etiologies of pleural effusions. Children with an effusion are usually older than 2 years of age but may be younger when the cause is a parapneumonic effusion (1,3).
A thoracentesis is a method to remove fluid or air from the pleural space (4). In this chapter, the discussion is limited to removal of fluid from the pleural space. A discussion relevant to the appropriate management and approach to the treatment of a pneumothorax is contained in Chapter 29.
Thoracentesis is indicated to remove pleural fluid that has caused respiratory embarrassment or to determine the etiology of the effusion (5). Depending on the volume and the characteristics of the pleural fluid, the procedure itself may be either definitive or temporizing. A tube thoracostomy is considered definitive therapy when an empyema is present or when continuous drainage is required, as in a traumatic hemothorax (4,5). The thoracentesis is most frequently performed on a semi-elective basis in a monitored setting. If the effusion is causing significant respiratory distress, however, a more urgent procedure is required.
Anatomy and Physiology
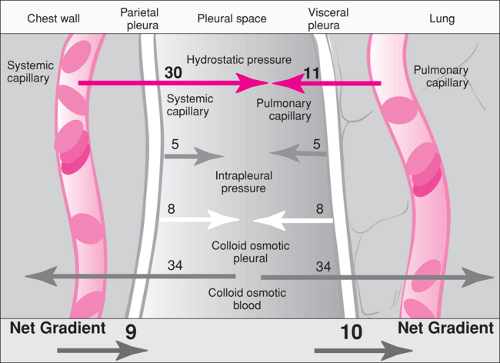
The pleural space is a potential space that exists between the visceral and parietal pleura. Normally this space contains less than 15 mL of pleural fluid (6). The formation of pleural fluid is controlled in part by the effect of Starling forces. The forces affecting net fluid movement across the pleura depend on the capillary and intrapleural hydrostatic pressures, the plasma and intrapleural oncotic pressures, and the capillary filtration coefficient. These forces are summarized in Figure 81.1. In a healthy person, the forces are such that protein-free extracellular fluid enters the pleural space from the parietal pleura and is absorbed at the visceral pleura (6,7,8). A small amount of protein in the pleural space leaks into the space from the pleural capillaries. The protein and approximately 10% of the pleural fluid leave the space through the rich lymphatic supply (7).
In the healthy state, no net accumulation of fluid is evident because of a balance between the Starling forces. A pleural effusion develops when various disease processes alter these forces and lead to net fluid accumulation (7,8). For example, in the hypoproteinemic state, a decrease in the serum oncotic pressure leads to a gradient favoring fluid transport to the pleural space. Pleural fluid collects in inflammatory diseases, such as pneumonia and serositis caused by a collagen-vascular disease, through an alteration in the capillary filtration coefficient. A pleural effusion also may develop if obstruction of the lymphatic drainage would occur, as with traumatic rupture of the thoracic duct.
The consequence of fluid collecting in the pleural cavity is an affect on normal respiratory physiology that may interfere with normal respiratory function. A large or rapidly expanding effusion may clinically cause respiratory embarrassment (9). The pleural fluid initially reduces the lung volume and consequently diminishes the vital capacity. If sufficient fluid accumulates, the vital capacity and the functional residual capacity decrease to the point where distal lung units collapse (i.e., atelectasis). The enlarging effusion also impedes diaphragmatic function and reduces chest wall compliance, which increases the work of breathing and makes deep sigh breaths more difficult. The ultimate effect is an impairment of alveolar gas exchange.
TABLE 81.1 Causes of Pleural Effusions | |
|---|---|
|
Indications
When the etiology of the pleural effusion is unknown or when respiratory embarrassment occurs because of the effusion, a thoracentesis should be performed. In certain circumstances, it may be reasonable to defer thoracentesis and institute therapy when the cause of the effusion is readily apparent. This would be the case in nephrotic syndrome with effusion or from left ventricular heart failure (10).
Clinical suspicion of a pleural effusion arises when a child presents with pain on inspiration, shortness of breath, dyspnea, decreased breath sounds, and dullness to percussion over the affected area. Occasionally, a pleural friction rub is heard.
An upright posteroanterior and lateral chest radiograph will confirm the clinical impression. When the effusion is small, the only radiographic abnormality may be a meniscus or “blunting” at the costophrenic angle on upright chest radiograph (11). This meniscus occurs with small or larger amounts of fluid (175 to 525 cc) (12). As the effusion enlarges, there is extension of the fluid up the lateral chest wall on the upright view (11). Large effusions may appear to be consolidation or “whiteout” of the entire lung and may include mediastinal shift away form the affected side (11). Ultrasound can be helpful in differentiating an effusion draping the lungs from a complete consolidation of the lung (11). Computerized tomography (CT) is also an excellent way to visualize pleural disease, distinguish and localize loculations, and aid in the differentiation of other causes of pleural effusions (13,14).
The appearance of a pleural effusion is somewhat different in recumbent films. If the fluid collection is less than
125 mL, a recumbent film may appear normal despite the presence of an effusion (15). Moderate size effusions appear as a homogeneous density in the lower lung fields, and as the collection enlarges, the entire lung field takes on a ground glass appearance (15). For all suspected pleural effusions, a lateral decubitus radiograph is valuable, as it helps identify questionable effusions and also aids in determining whether an effusion is free flowing or loculated (10). Loculated pleural fluid does not shift with position changes. When the fluid appears loculated, consideration should be given to a radiologically guided thoracentesis to ensure proper drainage and decrease the incidence of complications.
125 mL, a recumbent film may appear normal despite the presence of an effusion (15). Moderate size effusions appear as a homogeneous density in the lower lung fields, and as the collection enlarges, the entire lung field takes on a ground glass appearance (15). For all suspected pleural effusions, a lateral decubitus radiograph is valuable, as it helps identify questionable effusions and also aids in determining whether an effusion is free flowing or loculated (10). Loculated pleural fluid does not shift with position changes. When the fluid appears loculated, consideration should be given to a radiologically guided thoracentesis to ensure proper drainage and decrease the incidence of complications.
The most frequent indication for performing a thoracentesis is as a diagnostic aid. In adults, results of a thoracentesis give diagnostic or clinically useful information in over 90% of examinations when taken in the context of the clinical presentation (16). Before attempting the procedure, it may be helpful to consult a specialist in pediatric pulmonary medicine or infectious disease to review issues and determine which laboratory studies should be performed on the fluid.
Once fluid is obtained, the most useful information to be determined is whether the fluid is a transudate or an exudate. An exudate implies a breakdown of vascular integrity, as seen in infection, neoplasms, and other inflammatory processes. In contrast, a transudate is an ultrafiltrate of plasma. Table 81.1 categorizes the etiologies of pleural effusions as to whether they are associated with an exudative or a transudative process.
Although the laboratory diagnosis of an exudate is not absolute, the criteria established by Light and his colleagues are generally accepted to define an exudative process (10). Light et al. (17) defined an exudate as having any one of the three following characteristics:
A pleural/serum protein ratio greater than 0.5
A pleural/serum LDH ratio greater than 0.6
A pleural fluid LDH of more than two thirds the upper limits of normal for the serum LDH
A transudate will have none of these characteristics. In the initial study by Light et al., the sensitivity and specificity of the criteria approached 100%. Later studies on unselected populations have confirmed the high sensitivity of these criteria (18,19).
If initial studies indicate that the effusion is a transudate, some authorities have suggested that further laboratory evaluation of the fluid is costly and does not increase the diagnostic yield (19,20). These authorities suggest a two-step approach in which the initial laboratory studies are sent to determine whether an exudate or a transudate exists. During the initial collection of fluid, samples should be “held” so that they can be used at a later time. If the laboratory evaluation suggests an exudative process, the history and physical examination should guide further laboratory and diagnostic evaluation. For a transudate, the physician should treat the underlying disease process (5).
Pleural Fluid Laboratory Studies
This section reviews the potential laboratory studies that can be performed in the evaluation of pleural fluid. This is not meant to imply that all studies need to be sent for every patient. Clinical circumstances should guide the subsequent laboratory evaluation.
The gross appearance of the pleural fluid can be helpful. Transudates are often pale yellow. Milky white fluid suggests a chylothorax. Bloody fluid has been associated with neoplasms, pulmonary infarction, and trauma. This finding is tempered by the fact that 2 mL of blood into 1,000 mL of fluid can give a bloody appearance (21). A thick, purulent fluid is diagnostic of an empyema.
Pleural fluid is frequently sent for cytologic examination. Some authorities question the utility of cytologic examination for red and white blood cells (10,21,22). Light et al., in a prospective study of 182 patients, concluded that a red cell count of above 100,000/mm3 suggested neoplasm, pulmonary infarct, or trauma, but red cell counts between 10,000 and 100,000/mm3 were not specific for any disease process (21). They also concluded that the absolute white blood cell count is of limited utility due to a lack in both sensitivity and specificity. The differential count, however, may be helpful. A predominance of polymorphonuclear cells usually results from an acute inflammatory process such as pneumonia, pulmonary infarction, or a sympathetic effusion from pancreatitis. The presence of more than 50% lymphocytes strongly correlates with the presence of a malignancy or tuberculosis in patients with exudative effusions. The study did confirm the utility of cytologic examination for malignant cells, with a sensitivity of 77% when performed on one sample and of 90% when performed on three samples (21).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree