The Physiology of Puberty
Sarah A.B. Pitts
Catherine M. Gordon
Puberty is the natural life transition from childhood to adulthood. Change can be stressful, and puberty is no exception. As a girl proceeds through this time of change she and her parents, guardians, or teachers may have questions about what is normal versus abnormal. In order to gain the needed information, she may approach the family pediatrician, gynecologist, or other health care provider. An understanding of the physiology of puberty and menarche is essential for all health care providers so that they can dispense accurate information and help dispel myths. Once normal development is understood, the foundation is set for the diagnosis and management of precocious puberty (see Chapter 7) and menstrual abnormalities (see Chapters 8 to 11).
Hormonal Changes at Puberty
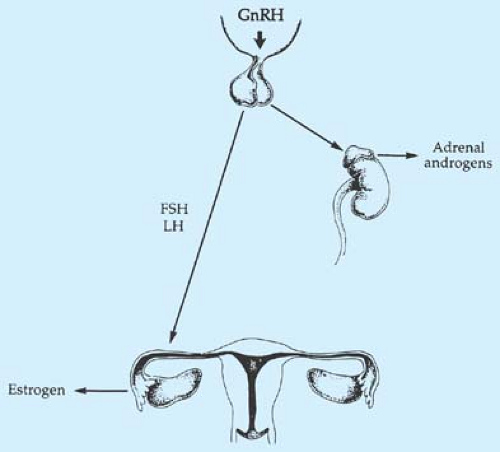
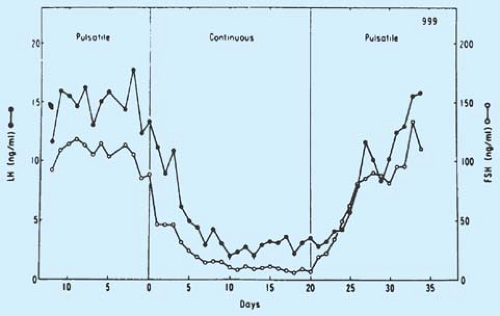
Normal female pubertal development requires the elaborate orchestration of the hypothalamic–pituitary–gonadal axis. This axis is active during the fetal and newborn periods, and then becomes quiescent during early childhood. What reawakens this axis at the time of puberty, resulting in accelerated growth and the appearance of secondary sex characteristics, is still under investigation. Before signs of puberty are visible, research has shown that inhibitory, stimulatory, and nutrition-dependent factors, such as γ-aminobutyric acid (GABA), neuropeptide Y, kisspeptin, and glutamate, begin to act on the hypothalamus, which then synthesizes and releases gonadotropin-releasing hormone (GnRH) in a pulsatile fashion (also referred to as luteinizing hormone–releasing hormone [LHRH]) (Fig. 6-1) (1). GnRH is a decapeptide with a serum half-life of 2 to 4 minutes that is released into the pituitary portal plexus and binds to surface receptors on anterior pituitary gonadotrophs, which synthesize and store the glycoprotein gonadotropins: follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Pulses of electrical activity have been recorded from the hypothalamus coincident with LH pulses (2). Pulsatile GnRH stimulation results in a pulsatile secretion of gonadotropins, as was demonstrated in the classic studies comparing pulsatile versus continuous administration of GnRH to oophorectomized rhesus monkeys (Fig. 6-2) (3). The pulsatile release of gonadotropins is thus responsible for ovarian stimulation and the resultant maturation of the germinal epithelium and synthesis of gonadal steroid hormones.
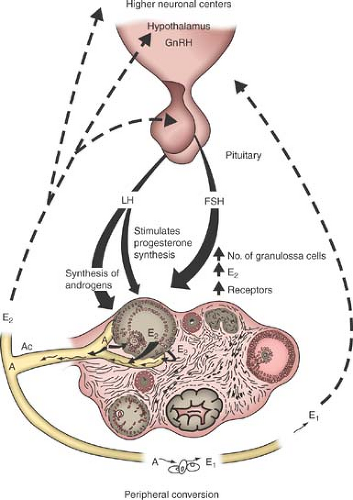
Sex steroids are produced in the follicles and theca cells of the ovary. In addition, the ovary produces insulin-like growth factor-1 (IGF-1), inhibin, activin, follistatin, and cytokines. These ovarian products exert a feedback effect upon gonadotropin secretion (Fig. 6-3). The feedback occurs both at the level of the hypothalamus, modulating the frequency and amplitude of GnRH release, and at the level of the pituitary, affecting the amount of LH and FSH released in response to GnRH pulses. Inhibin and activin are both disulfide-linked dimers composed of peptide subunits that are encoded by separate genes (4,5). Despite the structural similarity between these factors, they may function as antagonists, with activin stimulating and inhibin inhibiting FSH secretion (4,5). Follistatin is structurally unrelated to inhibin and activin, but acts as a regulator of the activin–inhibin system, decreasing FSH biosynthesis (4,5). Significant changes in these FSH-regulatory peptides accompany the onset of puberty (5). Serum inhibin A levels increase during midpuberty, while levels of inhibin B peak during midpuberty and decline thereafter (5). Serum inhibin B levels correlate positively with age many years before the clinical onset of puberty, suggesting increasing follicular activity in late prepuberty (5). During the pubertal period, inhibin B levels increase with breast development, suggesting high follicular activity before the onset of ovulatory menses (5). Levels of activin do not appear to vary across pubertal stages or across gender (5,6). Therefore, this factor does not appear to be involved in the endocrine modifications of pubertal development (6). Follistatin levels do not appear to change during puberty but increase with the establishment of regular menses (7). Ultimately, estrogen from the ovary suppresses gonadotropin secretion by negative feedback. Although both FSH and LH are released in pulses, LH pulses are recognizable by minute-to-minute serum measurements, as the half-life of LH is approximately 30 minutes compared with the 300-minute half-life of FSH.
The hypothalamic–pituitary–gonadal system is remarkably well developed at the time of birth. In fact, the hypothalamic
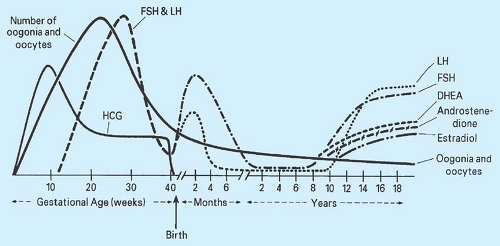
portal system is intact by 14 weeks of gestation. The negative feedback effect of gonadal steroids on the hypothalamus and pituitary is apparent by midgestation. The production of gonadotropins and ovarian sex steroids is important in the stimulation of germ cell division and follicular development. By 5 to 6 months of gestation, 6 to 7 million oocytes are present, and through the process of atresia, the neonate has approximately 1 million to 2 million oocytes at birth; by puberty only 0.3 million to 0.5 million oocytes remain (Fig. 6-4) (4). By 5 days after birth, gonadotropin levels rise sharply to levels considerably higher than those found in the prepubertal child, probably in response to the fall in placental estrogen. Transient rises in plasma FSH and estradiol are apparent in female infants, especially during the first 3 months of life. Thereafter, gonadotropin levels gradually fall to prepubertal levels, although FSH levels may not be maximally suppressed for 1 to 4 years. Girls have an elevated FSH/LH ratio compared with boys (8).
portal system is intact by 14 weeks of gestation. The negative feedback effect of gonadal steroids on the hypothalamus and pituitary is apparent by midgestation. The production of gonadotropins and ovarian sex steroids is important in the stimulation of germ cell division and follicular development. By 5 to 6 months of gestation, 6 to 7 million oocytes are present, and through the process of atresia, the neonate has approximately 1 million to 2 million oocytes at birth; by puberty only 0.3 million to 0.5 million oocytes remain (Fig. 6-4) (4). By 5 days after birth, gonadotropin levels rise sharply to levels considerably higher than those found in the prepubertal child, probably in response to the fall in placental estrogen. Transient rises in plasma FSH and estradiol are apparent in female infants, especially during the first 3 months of life. Thereafter, gonadotropin levels gradually fall to prepubertal levels, although FSH levels may not be maximally suppressed for 1 to 4 years. Girls have an elevated FSH/LH ratio compared with boys (8).
During the prepubertal childhood years, there is a down-regulation of the hypothalamic–pituitary system, with reduction of the amplitude and frequency of GnRH pulses (Fig. 6-5) and a decreased pituitary responsiveness (9). This inactivity appears to be in response to a central nervous system signal, with endogenous opiates serving as one of the primary inhibitory influences over GnRH secretion (10). In prepubertal girls, GnRH pulses continue to persist at low levels with enhancement during sleep. Levels of FSH and LH in Turner syndrome, which are markedly elevated in the neonatal period, are suppressed between the ages of 4 and 10 years, although the mean levels are higher than in normal children of similar ages. In some agonadal children between the ages of 5 and 11 years, basal levels of LH and FSH and responses to GnRH are comparable to those in normal prepubertal children; this similarity precludes a definitive diagnosis of gonadal failure by hormonal tests alone at this age (11).
The ovary increases in size during the prepubertal years and demonstrates evidence of active follicular growth and atresia. The vagina, which is approximately 4 cm long at birth, grows only 0.5 to 1.0 cm during early childhood but increases in length to 7.0 to 8.5 cm in late childhood. The uterus is about 2.5 cm long in infancy and grows to approximately 7.5 cm by adulthood. The corpus/cervix ratio is slightly <1:1; it reaches 1:1 at menarche and the adult ratio of 3:1 postmenarcheally.
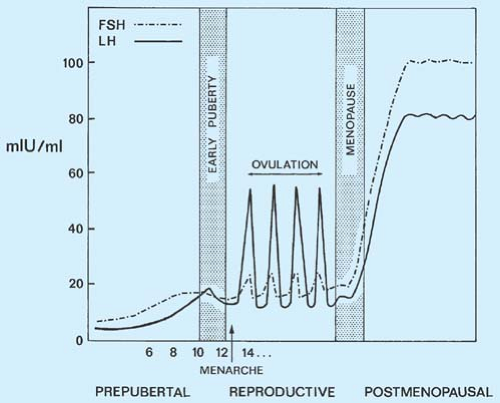 Figure 6-5. Gonadotropin levels from the age of 6 years to menopause. FSH, follicle-stimulating hormone; LH, luteinizing hormone. |
An early change associated with pubertal maturation is the secretion of adrenal androgens—dehydroepiandrosterone (DHEA), its sulfate (DHEAS), and androstenedione—between the ages of 6 and 8 years. Termed adrenarche, this process involves the regrowth of the zona reticularis (the zone that was large in the fetal adrenal cortex and regressed after birth) of the adrenal cortex with increases in activity of the microsomal enzyme P450c17. Recent data suggest that adrenarche is not the result of sudden rapid changes in adrenal enzymatic activity, but rather may be a gradual maturational process that begins in early childhood (12). In a group of pre-adrenarchal young girls on GnRH agonist therapy to suppress precocious puberty, gradual increases in 17,20–lyase activity and decreasing activity of 3β-hydroxysteroid dehydrogenase were seen, even in this
young cohort. Adrenal androgens continue to rise through ages 13 to 15 years and are primarily responsible for the appearance of pubic and axillary hair in girls (pubarche), although ovarian androgens, androstenedione and testosterone, also contribute (13,14). Local conversion of DHEAS to testosterone in the apocrine glands of the axillae causes body odor, and conversion within the sebaceous glands is responsible for the development of acne (14).
young cohort. Adrenal androgens continue to rise through ages 13 to 15 years and are primarily responsible for the appearance of pubic and axillary hair in girls (pubarche), although ovarian androgens, androstenedione and testosterone, also contribute (13,14). Local conversion of DHEAS to testosterone in the apocrine glands of the axillae causes body odor, and conversion within the sebaceous glands is responsible for the development of acne (14).
At approximately 8 years of age or thereafter, although no physical changes are present, GnRH secretion is enhanced, at first during sleep (15). There is a resultant increase in pituitary responsiveness and increased secretion of LH and FSH (16). This age-related rise in gonadotropins with initial sleep enhancement also occurs in girls with Turner syndrome, although markedly elevated (menopausal) levels of LH and FSH pulses are seen in this population (17).
It does not appear that ovarian sex steroids play a critical role in the onset of puberty, since patients with Turner syndrome experience adrenarche (the rise of adrenal androgens) and an age-related rise in gonadotropins. Prepubertal patients with Addison disease can experience normal gonadarche (the activation of the hypothalamic–pituitary–gonadal axis), but not adrenarche. Patients in whom precocious puberty begins before age 6 years typically exhibit gonadarche, but not adrenarche. Patients with constitutional delay of puberty frequently have delays in both adrenarche and gonadarche (18). Sex steroids are important for the development of functional feedback mechanisms (16).
During early puberty, there is a gradual augmentation of episodic peaks of LH and FSH during sleep. The onset of puberty is associated initially with a greater increase in LH pulse amplitude compared to frequency (19). There is a gradual increase in daytime pulsatility. LH pulsatility is GnRH dependent at all ages, while there is a decrease in GnRH regulation of FSH pulsatility as there is an increase in ovarian activity (4,20). Luteinizing hormone stimulates the ovarian theca cells to synthesize androgen precursors, and FSH increases the enzyme aromatase, which is responsible for the conversion of these precursors to estrogen. Estrogen peaks 10 to 12 hours after gonadotropin secretion (16). In addition, inhibin, activin, and follistatin interact in this complex regulatory system. These peptides are secreted in the highest levels by the gonads. In addition to the effect on pituitary FSH secretion, they also act locally in the gonad, affecting steroid biosynthesis and gametogenesis (7,21,22). The ovaries are marked by increased follicular growth and on ultrasonography may appear as “enlarged and multicystic” ovaries. These small “cysts” are normal and do not require aspiration or surgical intervention. As puberty progresses, the ovaries amplify the gonadotropin message and release greater amounts of sex steroids for a given level of gonadotropins.
Breast budding, estrogenization of the vaginal mucosa, and lengthening and enlargement of the uterus occur with estrogen exposure. The physiologic vaginal discharge of puberty is the result of the desquamation of epithelial cells and mucus from the estrogenized mucosa. The pubertal process is accompanied by a growth spurt; both growth hormone and sex steroids appear to contribute to the growth spurt. The levels of growth hormone and IGF-1 increase during puberty, largely dictated by the rise of serum estrogen (23,24,25,26). The effect of estrogen on growth hormone and growth is dose related; low doses of estrogen stimulate growth, growth hormone, and IGF-1, while high doses of estrogen decrease them. This observation led to the use, historically, of large pharmacologic doses of estrogen in an attempt to diminish final adult height in girls who were predicted to be excessively tall. In contrast, low doses of estrogen replacement in hypogonadal patients result in increased IGF-1 levels and growth. Since bone age, as determined by the Greulich and Pyle standards (27), correlates best with pubertal age, when an individual with delayed or advanced puberty is evaluated, it is important to obtain a radiograph of the wrist and hand for bone age interpretation. An increase in weight accompanies the growth spurt in normal girls, and body composition changes through late childhood and adolescence with a particularly apparent increase in percentage of body fat.
As puberty progresses, the levels of FSH and LH reached at night are gradually carried over into the waking hours until the sleep augmentation disappears. Even before menarche, circulating estrogen concentrations in pubertal girls have some cyclicity; eventually, these periodic fluctuations are sufficient to result in uterine bleeding. The first 1 to 2 years following menarche are often characterized by anovulatory menses (28,29,30,31,32). This time period coincides with the rapid growth of the uterus, vagina, fallopian tubes, and ovaries. With maturation, a mechanism known as the biphasic positive feedback system develops; in this system, a rise in plasma estrogen during the latter part of the follicular phase of the menstrual cycle triggers the surge of LH and FSH, which is responsible for ovulation. Historically, the change in the sensitivity of the feedback system was demonstrated by the use of clomiphene citrate, a nonsteroidal, agonist–antagonist estrogen, which when administered to prepubertal and early pubertal girls caused further suppression of gonadotropin levels (33,34,35). However, in late pubertal adolescents and adults, clomiphene causes a rise in gonadotropin levels and ovulation, a property that makes it useful as a fertility drug.
Leptin, a hormone secreted by adipose tissue, has been extensively studied and the balance of evidence suggests it plays a permissive role in reproductive function (36). In mouse models, leptin has been shown to reverse the sterility of leptin-deficient mice and accelerate the onset of puberty in normal mice (37). However, leptin levels have not been shown to change consistently with puberty in primates (38). In humans, serum leptin concentrations increase during childhood in both sexes (39). Although sufficient leptin concentrations appear to be associated with the initiation of puberty in girls with central precocious puberty (39), children with lipoatrophic diabetes who are, as a result, leptin deficient experience normal puberty, suggesting that leptin is only one factor involved in pubertal initiation (40). More likely, several hormones and neuropeptides work in concert to trigger the onset of puberty, and research in this area is ongoing.
Stages of Breast and Pubic Hair Development
In 1969, Marshall and Tanner (41) recorded the rates of progress of pubertal development of 192 English schoolgirls. These stages can be important guidelines in assessing whether an adolescent is developing normally.
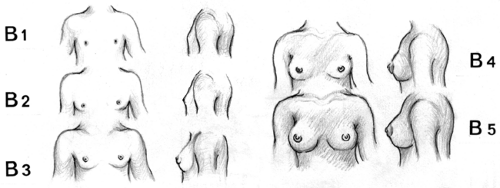
The Tanner stages—also termed Sexual Maturity Rating (SMR)—for breast development are as follows (Fig. 6-6) (15,41):
Stage B1 (preadolescent): elevation of the papilla only.
Stage B2 (breast-bud stage): elevation of the breast and papilla as a small mound, enlargement of the areolar diameter.
Stage B3: further enlargement of the breast and areola with no separation of their contours.
Stage B4: further enlargement with projection of the areola and papilla to form a secondary mound above the level of the breast.
Stage B5 (mature stage): projection of the papilla only, resulting from recession of the areola to the general contour of the breast.
Stage PH1 (preadolescent): The vellus over the pubes is not further developed than that over the anterior abdominal wall; no pubic hair.
Stage PH2: Sparse growth of long, slightly pigmented, downy hair, straight or only slightly curled, appearing chiefly along the labia.
Stage PH3: Hair darker, coarser, and curlier spreads to extend sparsely over the junction of the pubes.
Stage PH4: Hair adult in type spreads over the mons pubis but not to the medial surface of the thighs.
Stage PH5 (mature stage): Hair adult in quantity and type spreads to the medial surfaces of the thighs; distribution in an inverse triangle forms the classic feminine pattern.
The mean age of each pubertal stage for British girls is shown in Fig. 6-8. The ages of normal sexual development for American girls are shown in Table 6-1 (42).
There is growing concern regarding a secular trend toward an earlier age of pubertal onset in girls (43,44). Experts believe that there are sufficient data to support a trend in earlier onset of breast budding in girls and earlier age of menarche. The data are not sufficient to support a corresponding trend in boys. From 1940 to 1994, there has been a 2.5- to 4-month decrease in age of menarche. The evidence points to elevated levels of body fat in prepubertal girls and to endocrine-disrupting chemicals in the environment as contributing factors, both of which have public health implications (37,43,45).
The first sign of puberty in 85% to 92% of white girls is breast budding, which may initially be unilateral. Some girls pass from stage B3 directly to stage B5, and some remain in stage B4. Developmental stages are assessed most accurately by observation of sequential changes in an individual girl by one clinician. In the series of Marshall and Tanner (41), the mean interval from stage B2 to stage B5 was 4.2 years. Pubic hair development usually lags by about 6 months and appears at an average age of 11 to 12 years. Pubic hair as the first sign of development may be a normal variant (especially in those of African American descent), but in some girls, it may reflect an excess of androgens that later may cause hirsutism and menstrual irregularity (see Chapters 10 and 11). The mean interval from stage PH2 to stage PH5 is 2.7 years. Generally, pubic hair will not advance beyond stage PH2 or PH3 in the absence of gonadal sex steroids. The beginning of breast development usually corresponds to the onset of the growth spurt. The timing of these events is variable, but 98.8% of girls have the first signs of sexual development between the ages of 8 and 13 years.
Tanner stage 2 breast development may be difficult to identify in obese prepubertal girls, and Biro (46) has suggested the utility of the Garn–Falkner system for staging areola. With this system, four areolar stages are identified on the basis of areolar diameter, pigmentation, and contour, with areolar stage 1 being prepubertal. Areolar stage 2 displays palpable subareolar
tissue, an increase in size and pigmentation of the areola, and little development of the papilla. Areolar stage 3 has a further increase in size and pigment of the areola, with separation of the areola and papilla from the contour of the breast. Areolar stage 4 has an elevation of the papilla and regression of the areola, with a mature size and color of the areola (not all women develop stage 4). Comparison is made to staging plates. Biro (46) reported that 9 of 15 girls in breast Tanner stage 2 were in areolar stage 1.
tissue, an increase in size and pigmentation of the areola, and little development of the papilla. Areolar stage 3 has a further increase in size and pigment of the areola, with separation of the areola and papilla from the contour of the breast. Areolar stage 4 has an elevation of the papilla and regression of the areola, with a mature size and color of the areola (not all women develop stage 4). Comparison is made to staging plates. Biro (46) reported that 9 of 15 girls in breast Tanner stage 2 were in areolar stage 1.
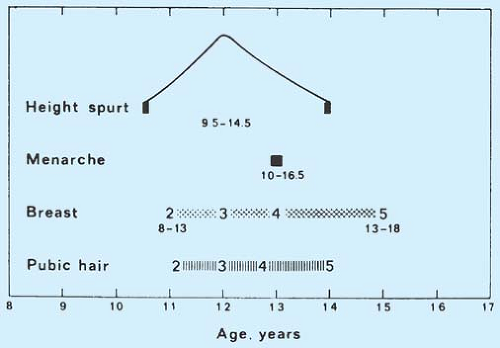 Figure 6-8. The sequence of events at puberty in British females. (Adapted from Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291.) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree