Amenorrhea in the Adolescent
S. Jean Emans
Amy DiVasta
The distinction between pubertal delay and amenorrhea can be somewhat artificial because many problems that cause pubertal delay can also cause amenorrhea. In the girl presenting with primary amenorrhea, the clinician needs to assess whether there is a hypothalamic–pituitary–ovarian (HPO) axis abnormality or a genital anomaly. The differential diagnosis of amenorrhea (see Fig. 9-1) may range from constitutional delay, eating disorders, chronic disease, stress, and central nervous system (CNS) tumors to thyroid and adrenal disorders to primary ovarian insufficiency (POI), pregnancy, genital anomalies, and polycystic ovary syndrome (PCOS). A stepwise evaluation using history, growth charts, physical examination, and limited laboratory tests will rule in and rule out the major causes of menstrual disorders in adolescent girls.
The mean age of menarche in the United States has decreased significantly over the past 100 years, but less in the past five decades (see also Chapter 6). In the National Health and Nutrition Survey III (NHANES III, 1988–1994), the mean age of menarche was 12.1 years for black girls, 12.2 years for Mexican American girls, and 12.7 years for white girls (1). Chumlea and colleagues found the mean age of menarche was 0.34 year earlier than in 1973 (2). Comparing data from NHANES III with National Household Education Surveys (NHES) data (1966–1970), Sun and colleagues (3) found that only Mexican American girls had evidence of earlier stage 2 and 4 breast and pubic hair. In general, higher body mass index (BMI) has been associated with earlier onset of breast budding and earlier age of menarche, with different studies finding particular racial/ethnic groups to be affected (4,5). A low BMI is associated with amenorrhea in athletes and in girls with eating disorders.
Fewer than 10% of girls have menarche before age 11, and conversely, 90% have begun to menstruate by age 13.75 years (1,2). Absence of menarche by age 15 years is thus termed primary amenorrhea. Only 3 in 1000 girls will experience menarche after 15½ years. In fact, menstruation has been termed “a vital sign” that should be used to assess normal development in girls (6,7).
In assessing the individual patient, the clinician needs to keep in mind the normal stages of puberty. Although most girls have the onset of menses within 2 to 2½ years of the beginning of breast development (thelarche), the range is variable, and menarche may not occur in an individual girl for 3 or 4 years. For example, if the healthy 15-year-old began her pubertal development at the age of 13 years, she can usually be reassured that she can expect her menarche by the age of 15 or 16 years, 2 to 3 years after the onset of secondary sexual characteristics. The patient should be observed for a reassuring steady progression of growth and development. A halt in maturation signifies the need to do a thorough endocrine evaluation. In contrast, the girl who is age 15 years and started her development at age 11 but has not had her menarche warrants an evaluation to determine the cause, including a pelvic examination or ultrasound (and, if needed, pelvic magnetic resonance imaging [MRI]) to exclude a genital tract anomaly.
Secondary amenorrhea is usually defined as 3 months of amenorrhea, but timing an evaluation depends on the age and the possibility of pregnancy, a frequent cause of amenorrhea in teens. Denial of intercourse is common among teenagers, and young adolescents may not understand their physiology or may have become pregnant by rape or incest. Thus, a pregnancy test should be done whenever an adolescent expresses concern about a menstrual period being late, even if only by 2 or 3 weeks, and as a routine part of evaluation of secondary amenorrhea. Further evaluation of amenorrhea is important for adolescents who have had 3 to 6 months of amenorrhea without an obvious cause (such as dieting or weight loss), persistent oligomenorrhea (irregular cycles >40 days apart), estrogen deficiency, or androgen excess (hirsutism, acne). Even in girls with weight loss, an evaluation should be undertaken with persistent amenorrhea to rule out other causes not initially suspected.
Although many clinical series focus on unusual causes of amenorrhea, school-based studies have highlighted the role of disordered eating and weight change in the etiology of amenorrhea in teens (8,9). Abnormal eating patterns and weight change are the most important risk factors for missing three consecutive menses in the previous year (Tables 9-1 and 9-2). In a study involving 270 schools, menses less often than once a month were 1.6 times more likely in girls who vomited 1 to 3 times per month for weight control and 3.2 times more likely in girls who vomited ≥1 time per week (9).
Patient Assessment
A careful history and physical examination are essential for the evaluation of the adolescent girl with amenorrhea. As noted in Chapter 8, the history should include an assessment of genetic and familial disorders, including developmental delay and retardation (associated with fragile X); age of menarche and fertility of mother, sisters, and other relatives; neonatal history; previous surgeries; treatments for malignancies, autoimmune disorders, or endocrinopathies; review of systems; and rate of pubertal development. The review of systems should focus on headaches, disordered eating, weight changes, sexual activity, stress, gastrointestinal symptoms, galactorrhea (nipple discharge), athletic participation, medications, acne, and hirsutism.
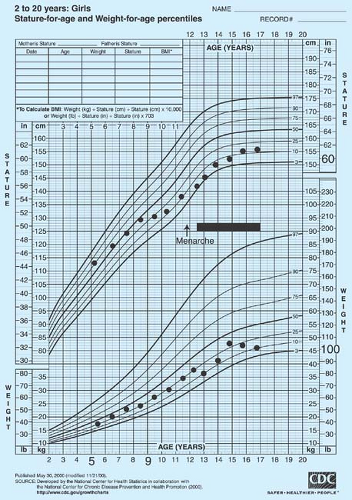
Assessment of the pattern of growth can yield valuable information (Figs. 9-2 and 9-3). Failure of growth in height for several years may occur with Crohn or celiac disease (see Fig. 27-1 in Chapter 27) or an acquired endocrine disorder. Cessation of growth may also indicate that the adolescent has reached a bone age of 15 years and her epiphyses have fused. In conditions associated with poor nutrition, such as anorexia nervosa, celiac disease, and inflammatory bowel disease, the patient is typically underweight for her height. In contrast, patients with acquired
hypothyroidism and cortisol excess (iatrogenic or Cushing syndrome) are typically overweight for height. Obtaining a radiograph of the wrist and hand for bone age can be helpful in assessing the amount of growth remaining in patients with primary amenorrhea and short stature (see Appendix 2 for estimates of final height and Chapter 8 [p. 127] for discussion of calculation of midparental height). Menarche is more closely linked to bone age than to chronologic age. Girls with classic congenital adrenal hyperplasia (CAH) may have early puberty or have delayed
menarche or secondary amenorrhea with advanced bone age from poor control of elevated androgens.
hypothyroidism and cortisol excess (iatrogenic or Cushing syndrome) are typically overweight for height. Obtaining a radiograph of the wrist and hand for bone age can be helpful in assessing the amount of growth remaining in patients with primary amenorrhea and short stature (see Appendix 2 for estimates of final height and Chapter 8 [p. 127] for discussion of calculation of midparental height). Menarche is more closely linked to bone age than to chronologic age. Girls with classic congenital adrenal hyperplasia (CAH) may have early puberty or have delayed
menarche or secondary amenorrhea with advanced bone age from poor control of elevated androgens.
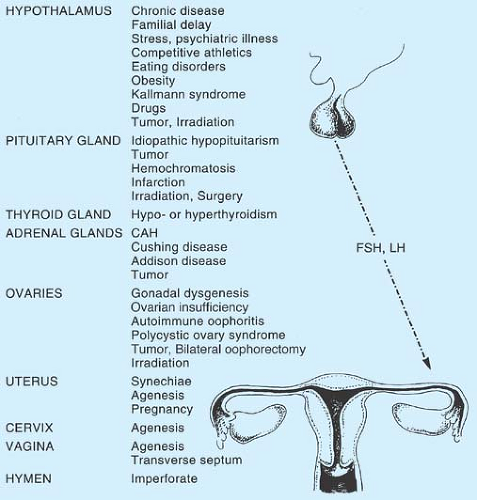 Figure 9-1. Etiology of primary amenorrhea. CAH, congenital adrenocortical hyperplasia; FSH, follicle-stimulating hormone; LH, luteinizing hormone. |
Table 9-1 Percent of Adolescent High School Girls who Reported Missing Three Consecutive Menses During the Past Year (n = 2156) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Table 9-2 Risk Factors for Secondary Amenorrhea (3 mo) in 2588 High School Girls | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Physical Examination
The physical examination of the girl with primary amenorrhea involves a general assessment including height and weight, calculation of BMI, blood pressure, palpation of the thyroid gland, and Tanner (Sexual Maturity Rating [SMR]) staging of breast development and pubic hair. The breasts should be compressed gently to assess for the presence of galactorrhea, since patients frequently do not report this finding. Midline facial defects may be associated with hypothalamic–pituitary dysfunction. Renal and vertebral anomalies and hernias (inguinal) may be associated with müllerian malformations. The somatic stigmata of Turner syndrome may be a clue to this diagnosis (p. 130). A brief neurologic examination may include an assessment of the ability to smell, funduscopic examination, and screening visual field tests by confrontation. Sparse or absent pubic and axillary hair in the presence of normal breasts and primary amenorrhea should increase suspicion of androgen insensitivity. Panhypopituitarism and adrenal insufficiency also result in sparse pubic and axillary hair. The presence of hirsutism, acne, acanthosis nigricans, and amenorrhea most likely indicate a diagnosis of PCOS (see Chapter 11).
Recent weight loss and decrease in BMI may provide a potential explanation for the amenorrhea. Although low-weighted girls may have normal menses, the determination of target BMI in the girl with amenorrhea may aid in setting realistic goals for the future (see p. 145). While amenorrhea has been associated with low body fat, methodologic issues abound in clinically estimating percentage of body fat in contrast to using imaging such as dual-energy X-ray absorptiometry (DXA) (10,11,12,13,14). A clinical estimate of percentage of body fat, if helpful for the evaluation, can be obtained by measuring four sites of skinfold thickness (triceps, biceps, subscapular, and suprailiac) using calipers and comparing the results with the tables and equations given in Appendix 3.
The gynecologic assessment involves first inspection of the external genitalia to determine if the girl has clitoromegaly and a normal hymenal opening and whether there is estrogen effect on the hymen and anterior vagina. Normal breast development and an estrogenized vagina imply that the ovaries are making estrogen. The degree of estrogenization noted at the time of the initial examination can often help the clinician decide the extent of the workup indicated. The finding of a reddened, thin vaginal mucosa is consistent with estrogen deficiency and is more worrisome than the finding of an estrogenized, pink, moist vaginal mucosa. However, the finding of estrogen effect does not exclude pathologic diagnoses.
In the girl with normal pubertal development and primary amenorrhea, assessment of internal genital structures is essential
to exclude a genital anomaly. The presence or absence of pubic hair should be noted. Although an imperforate hymen is typically detected in the newborn nursery or during early childhood, it is sometimes not diagnosed until the patient is an adolescent. A bulging bluish-tinged hymen may be noted in the adolescent with an imperforate hymen and blood-filled vagina (hematocolpos) (see Chapter 12). To assess patency and length and width of the vagina, a saline-moistened cotton-tipped applicator (or Calgiswab) can be gently inserted into the hymenal opening and moved from side to side of the vagina (the vaginal sample can also be used for a maturation index to assess estrogenization, if desired; see section below). The patient with a transverse vaginal septum or vaginal, cervical, and uterine agenesis has normal-appearing external genitalia. However, despite the normal external appearance, in a patient with vaginal agenesis, a cotton-tipped applicator can be inserted only 0.5 to 2 cm. In an estrogenized patient with normal vaginal length and width, a gentle one-finger examination of the vagina will allow palpation of the cervix and uterus by bimanual vaginal–abdominal examination with the patient in the lithotomy position. If needed, visualization of the cervix is usually possible with a small Huffman speculum. In the nonobese teenager, a simple rectoabdominal examination in the dorsal supine position also can be used to confirm the presence of the cervix and the uterus. In the unestrogenized adolescent, the knee–chest position (see Chapter 1) can be used, just as in the prepubertal child, to visualize the vagina and the cervix, although the position is likely to be experienced as embarrassing for most teens.
to exclude a genital anomaly. The presence or absence of pubic hair should be noted. Although an imperforate hymen is typically detected in the newborn nursery or during early childhood, it is sometimes not diagnosed until the patient is an adolescent. A bulging bluish-tinged hymen may be noted in the adolescent with an imperforate hymen and blood-filled vagina (hematocolpos) (see Chapter 12). To assess patency and length and width of the vagina, a saline-moistened cotton-tipped applicator (or Calgiswab) can be gently inserted into the hymenal opening and moved from side to side of the vagina (the vaginal sample can also be used for a maturation index to assess estrogenization, if desired; see section below). The patient with a transverse vaginal septum or vaginal, cervical, and uterine agenesis has normal-appearing external genitalia. However, despite the normal external appearance, in a patient with vaginal agenesis, a cotton-tipped applicator can be inserted only 0.5 to 2 cm. In an estrogenized patient with normal vaginal length and width, a gentle one-finger examination of the vagina will allow palpation of the cervix and uterus by bimanual vaginal–abdominal examination with the patient in the lithotomy position. If needed, visualization of the cervix is usually possible with a small Huffman speculum. In the nonobese teenager, a simple rectoabdominal examination in the dorsal supine position also can be used to confirm the presence of the cervix and the uterus. In the unestrogenized adolescent, the knee–chest position (see Chapter 1) can be used, just as in the prepubertal child, to visualize the vagina and the cervix, although the position is likely to be experienced as embarrassing for most teens.
Pelvic ultrasonography can be obtained for confirmation of the examination findings or if the patient is not comfortable with a modified pelvic examination. If a pelvic ultrasound is obtained in a poorly estrogenized girl, the radiology report should be interpreted with caution because the uterus may be small and difficult to visualize and thus may be assumed to be absent. If questions are raised at the time of the initial evaluation, the ultrasound can be repeated in a pediatric center accustomed to the appearance of the prepubertal uterus or in some cases after a course of estrogen therapy. An MRI is important if a congenital anomaly or obstructed genital tract is found; laparoscopy is sometimes needed to better define uterine structures in women with vaginal agenesis (15) (see Chapter 12).
The history and physical examination of the girl with secondary amenorrhea or oligomenorrhea is similar, except that a genital anomaly is unlikely. Pregnancy needs to be ruled out
early in the assessment (rarely, pregnancy can also cause primary amenorrhea). Depending on whether the girl is sexually active or is comfortable with a pelvic examination, the gynecologic assessment may include a speculum examination, a bimanual vaginal–abdominal or rectoabdominal examination, or a pelvic ultrasound.
early in the assessment (rarely, pregnancy can also cause primary amenorrhea). Depending on whether the girl is sexually active or is comfortable with a pelvic examination, the gynecologic assessment may include a speculum examination, a bimanual vaginal–abdominal or rectoabdominal examination, or a pelvic ultrasound.
Laboratory Tests
Initial laboratory screening tests include a urine or serum human chorionic gonadotropin (HCG) test, a complete blood count (CBC), urinalysis, and serum levels of thyroid-stimulating hormone (TSH) and/or free thyroxine (free T4), follicle-stimulating hormone (FSH), and prolactin. Luteinizing hormone (LH) is also often drawn with the initial panel. These should be drawn before any hormones are given, including a progestin challenge, so that levels are not altered. The HCG result should be obtained early in the workup, since very rarely pregnancy will occur without menarche. In girls with unexplained amenorrhea or possible symptoms of chronic illness, an erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) along with a chemistry panel (blood urea nitrogen [BUN], creatinine, electrolytes, liver function tests, glucose, calcium) and screen for celiac disease are helpful.
The differential diagnosis can usually be divided on the basis of FSH levels into categories of hypergonadotropic hypogonadism (high FSH levels >30 mIU/mL indicating POI) and hypogonadotropic hypogonadism (low or normal levels of FSH indicating hypothalamic or pituitary dysfunction) (16) (Fig. 9-4). Unless a high FSH level is expected because of prior radiation or chemotherapy, a single high FSH should be repeated in 2 to 4 weeks along with an estradiol level (which is typically <50 pg/mL) before a definitive statement is made about primary ovarian insufficiency (POI). Even FSH levels >10 mIU/mL except at the midcycle preovulatory surge may suggest possible ovarian dysfunction. Of note, while not yet used clinically in adolescents, levels of anti-müllerian hormone (AMH), secreted by the granulosa cells, are a marker of the ovarian follicle pool and are low in POI.
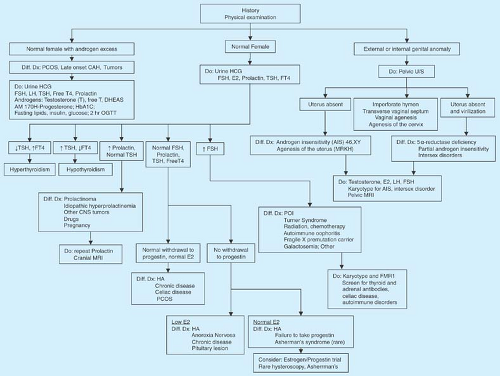 Figure 9-4. Evaluation of amenorrhea. E2, estradiol; HA, hypothalamic amenorrhea; POI, primary ovarian insufficiency. |
Low to normal levels of FSH (and LH) imply a CNS cause, such as hypothalamic dysfunction, which may be primary or secondary due to chronic disease, endocrinopathy, stress, an eating disorder, exercise, or, rarely, a CNS tumor. The FSH and LH levels can be better interpreted if the clinician knows when the next menstrual period occurs in the oligomenorrheic patient.
Checking to ensure that the patient did not have menstrual bleeding 2 weeks after the sample was drawn allows correct interpretation of the results. If the LH level is drawn during the midcycle, ovulatory LH surge, the concentrations can be three times normal baseline levels. An increased LH level with a normal FSH level is often found in girls with PCOS but is not definitive (see Chapter 11).
Checking to ensure that the patient did not have menstrual bleeding 2 weeks after the sample was drawn allows correct interpretation of the results. If the LH level is drawn during the midcycle, ovulatory LH surge, the concentrations can be three times normal baseline levels. An increased LH level with a normal FSH level is often found in girls with PCOS but is not definitive (see Chapter 11).
In addition to the visual appearance of the vaginal mucosa and secretions on physical examination, several methods can provide information about the patient’s estrogen status—progestin withdrawal, vaginal smear, or serum estradiol level—but all have their limitations. A normal test does not exclude the possibility of a pathologic diagnosis. The progestin challenge provides an estimate of the estrogen concentration and confirms the presence of an estrogen-primed uterus. After confirming that the patient is not pregnant (and drawing the hormone tests above), the clinician prescribes a progestin challenge with oral medroxyprogesterone (5 or 10 mg once a day) or micronized progesterone (200 to 300 mg once a day) for 10 days (some prefer 5 days). A positive response to the progestin challenge is normal withdrawal bleeding (3- to 5-day menses) typically occurring 2 to 3 days after the end of the progestin dose, but possibly starting up to 10 days later. The response to the progestin challenge tends to correlate with estradiol level; in one study (17), the mean estradiol concentration was 60 pg/mL in 63 women who had withdrawal bleeding compared to a mean value of 18 pg/mL in 27 women who had no withdrawal response. A 10-day course of micronized progesterone, 300 mg, induced withdrawal bleeding in 90%. of women with a screening estradiol >50 pg/mL (18). The progestin withdrawal test is not helpful in the patient with delayed development or in the patient who clearly appears estrogen deficient on clinical examination (e.g., a girl with anorexia nervosa), as bleeding will not occur. Although a normal menstrual flow in response to progesterone suggests adequate estrogen levels, up to 50% of patients with a variety of pathologic diagnoses such as prolactinomas and POI may have a normal response.
A vaginal smear obtained during the genital examination can be evaluated for maturation index (p. 16) to aid in assessing estrogen effect over time. Lastly, while the serum estradiol level provides information about a single point in time, it may not provide information on overall estrogen status. Most girls with POI will have an estradiol concentration <50 pg/mL; however, even normal adolescents in the first few days of a cycle may have low estrogen levels. Similarly, girls with estrogen deficiency may have a low normal level. Thus, while the combination of an estradiol level and a progestin challenge may provide the clinician with useful information, these tests should not change or delay obtaining screening tests for amenorrhea. A pelvic ultrasound can also provide information regarding the thickness of the endometrial stripe and the size of the uterus, providing further evidence of estrogen effect on the reproductive organs.
In the apparently estrogenized patient with normal gonadotropin and prolactin levels and failure to have withdrawal bleeding from progesterone, the presence of a normal endometrium can be established by obtaining an ultrasound and then by giving combination estrogen and progestin (e.g., 21 days of an oral contraceptive with 35 μg ethinyl estradiol [EE] or conjugated estrogens 0.625 to 1.25 mg or estradiol 1 to 2 mg on days 1 to 25 and medroxyprogesterone 10 mg on days 16 to 25) for two to three cycles. However, it should be noted that a diagnosis of uterine synechiae (Asherman syndrome) is sufficiently rare in the absence of a history of uterine infections, abortion, or dilation and curettage (D&C) that this part of the evaluation can be omitted until the many other possible diagnoses for amenorrhea are considered. Other rare diagnoses resulting in disruption of normal endometrium are tuberculosis and schistosomiasis. Another explanation for the lack of withdrawal flow in the well-estrogenized normal adolescent girl is her failure to take the progestin for the withdrawal challenge.
As noted in Chapter 6, the development of normal bone mineral density (BMD) is a major concern for adolescents with amenorrhea. Measurement of BMD at the lumbar spine and total hip is important in girls with more than 6 to 12 months of hypoestrogenic amenorrhea in order to provide baseline information on bone mass and potential bone loss. The center performing the BMD density measurements should have software that can provide age-matched standards for comparison. For girls with significant pubertal delay, the results should be corrected for bone age.
The decision of whether or not to obtain CNS imaging studies in the patient with normal serum FSH and prolactin values can be a clinical dilemma. Most pituitary tumors causing primary or secondary amenorrhea in adolescents secrete prolactin, interrupt normal prolactin inhibition sufficiently to cause a mild to moderate elevation of prolactin, or are associated with other signs or symptoms. Given the likelihood that most adolescents have hypothalamic amenorrhea because of stress, athletics, or weight changes, and since nonfunctioning tumors that cause amenorrhea are extremely rare, an MRI scan is generally reserved for the evaluation of the patient with interrupted puberty, an elevated prolactin level, and/or neurologic signs or symptoms. An MRI may also be indicated in the evaluation of the older adolescent or young adult woman who has persistent amenorrhea with no obvious etiology.
Patients with amenorrhea (primary or secondary) and hirsutism usually have PCOS or, more rarely, late-onset CAH (see Chapter 11) or Cushing syndrome. Patients with true virilization are rarer and may have PCOS, late-onset CAH, an ovarian or adrenal tumor, mixed gonadal dysgenesis, an incomplete form of androgen insensitivity, 5α-reductase deficiency, gonadal dysgenesis (with virilization), or true hermaphroditism (see Chapters 3, 11, and 12).
Hypogonadotropic Hypogonadism
The etiologies for hypogonadotropic hypogonadism are numerous and include chronic illness (especially those associated with poor nutrition such as Crohn disease, celiac disease, cystic fibrosis, and sickle cell disease), undernutrition, stress, eating disorders, excessive exercise, medications, genetic syndromes, hypothalamic disorders such as Kallmann syndrome and tumors, and pituitary problems such as prolactinomas, non-prolactin-secreting tumors (19) (which may secrete FSH, LH, or the α-subunit of the glycopeptide hormones), infarction, and infiltrative disease (see Chapter 8). Kallmann syndrome is caused by several mutations including the KAL gene Xp22.3, which is associated with anosmia; other genetic mutations may or may not have anosmia. Hydrocephalus due to aqueductal stenosis can present with headaches and amenorrhea during adolescence (20). Acquired hypopituitarism can result from
head trauma, postpartum shock and pituitary necrosis (Sheehan syndrome), pituitary infarction (e.g., sickle cell disease), and an autoimmune process (21). Empty sella can be associated with hypothalamic pituitary dysfunction; in one series of children with multiple pituitary deficiencies, one-third had an empty sella (22). A normal-sized sella that is empty may be associated with pituitary hypoplasia, an unrecognized pituitary insult, dysfunction of the hypothalamus or higher centers resulting in diminished pituitary growth, or herniation of cerebrospinal fluid through a congenitally incomplete sellar diaphragm (23).
head trauma, postpartum shock and pituitary necrosis (Sheehan syndrome), pituitary infarction (e.g., sickle cell disease), and an autoimmune process (21). Empty sella can be associated with hypothalamic pituitary dysfunction; in one series of children with multiple pituitary deficiencies, one-third had an empty sella (22). A normal-sized sella that is empty may be associated with pituitary hypoplasia, an unrecognized pituitary insult, dysfunction of the hypothalamus or higher centers resulting in diminished pituitary growth, or herniation of cerebrospinal fluid through a congenitally incomplete sellar diaphragm (23).
Girls with poorly controlled diabetes mellitus, depression, psychological problems, and substance abuse may have irregular menses. Self-imposed caloric restriction and intermittent dieting are common among adolescent girls, many of whom view themselves as overweight. Even in normal-weighted girls, mild dieting, dietary fat restriction, and bingeing/purging are associated with irregular menses; these behaviors may be initially denied by the patient (9,24,25,26,27). In a study of 16 participants, women with hypothalamic amenorrhea took in only 16.3% of calories from fat compared to 31.6% in normal-cycling controls (27). Similarly, weight loss even in obese patients who still remain significantly overweight for height can result in amenorrhea.
The term hypothalamic amenorrhea refers to a spectrum of menstrual dysfunction, ranging from prolonged amenorrhea and severe estrogen deficiency from anorexia nervosa to minor changes in endocrine function due to stress, some of which may have a genetic basis (28,29,30,31,32,33). In a study of 49 adult women with hypothalamic amenorrhea, LH pulses varied from apulsatile (8%) to low frequency/low amplitude (27%), low amplitude/normal frequency (8%), low frequency/normal amplitude (43%), and normal frequency/normal amplitude (14%) (34). The abnormal pattern of gonadotropin-releasing hormone (GnRH) and gonadotropin secretion varies over time in the same woman. In addition, there is often overactivity of the hypothalamic–pituitary adrenal axis, with increased secretion of corticotrophin-releasing hormone (CRH), adrenocorticotropin hormone (ACTH), cortisol, and endogenous opioids (28,35,36,37). Low energy availability may lead to growth hormone resistance so that growth hormone levels are increased and insulin-like growth factor-1 (IGF-1) decreased. There are also other factors including leptin, ghrelin, and neuropeptide Y that affect GnRH regulation (33,38,39).
Special sections below review some of the relevant literature on causes of hypogonadotropic hypogonadism in adolescents: eating disorders, the female athlete triad, and prolactinomas. A normal physiologic delay in menarche is a diagnosis of exclusion and requires a careful medical evaluation before watchful waiting or hormonal therapy is undertaken.
Eating Disorders
Eating disorders are prevalent in contemporary society. Some adolescents pursue thinness to the extreme of causing delayed development, delayed menarche, and secondary amenorrhea. Adolescents with bulimia and normal weight for height may have regular or irregular menses. The lifetime risk for anorexia nervosa (AN) in women has been estimated at 0.3% to 1%; a higher percentage of women have subclinical AN. The etiology of AN is multifactorial and includes genetic influences; personality traits; anxiety, depression, and obsessive compulsive disorder; family history of affective disorders; and family, peer, coach, sports, and media pressures related to the “ideal” of thinness (40,41). The onset of AN has a bimodal distribution. Early adolescents may have the onset of AN at a time of pubertal maturation, a compensatory increase in weight, and body image concerns. A second peak occurs during later adolescence (ages 17 to 18 years) at a time of separation and choices about jobs and college. Girls with AN are typically preoccupied with thoughts of food and often feel inadequate, experiencing a pervasive lack of control. Caloric deficits occur as a result of restricted caloric intake and excessive energy expenditure (exercise). Other adolescents may binge and then purge by self-induced vomiting or by abuse of laxatives. Young women with other medical problems such as Turner syndrome or diabetes mellitus may also develop eating disorders. The Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) criteria for the diagnosis of anorexia nervosa have included refusal to maintain body weight (weight loss with weight <85% of expected body weight for age or failure to gain during a period of normal growth), intense fear of gaining weight, disturbance in body image, and amenorrhea (absence of three consecutive cycles) (42). However, the proposed DSM-V criteria are likely to eliminate amenorrhea and to avoid setting numerical measures for weight or BMI but instead focus on restrictive behaviors leading to a markedly low body weight, fear of gaining weight, and disturbance in self-evaluation of weight. Treating patients early in the course of the illness, even before these criteria are met, may yield a better prognosis. Since these girls are frequently secretive about their eating patterns, it is useful for the clinician to develop some techniques for eliciting an accurate history, such as saying, “Where would you like your weight to be? How hard do you have to work to keep your weight where you want it to be? Have you ever used vomiting or medicines to control your weight?”
A multitude of medical problems are associated with eating disorders, including dehydration and electrolyte imbalance (43). Vital signs are usually depressed in girls with anorexia nervosa, with low temperature, blood pressure, and pulse rate. Other signs include dry skin, lanugo, bruises, edema (during refeeding), murmurs, abdominal bloating, constipation, cold intolerance, and stress fractures. Laboratory abnormalities in AN include low FSH, LH, and estradiol. While TSH and T4 are usually normal, triiodothyronine (T3) is often low (“sick euthyroid syndrome”). The differential diagnosis of anorexia nervosa includes inflammatory bowel disease, celiac disease, Addison disease, hyperthyroidism, malignancy, diabetes mellitus, depression, and CNS tumors.
Substantial deficits in bone density may occur and may be irreversible and associated with low weight, low lean body mass, low fat intake, estrogen deficiency, hypercortisolism, low dehydroepiandrosterone sulfate (DHEAS), and IGF-1 deficiency (44,45,46,47,48,49,50,51). DXA measurements of the spine and hip are significantly related to lean body mass and are lower in women with anorexia nervosa than hypothalamic amenorrhea (44). Girls with anorexia nervosa often become amenorrheic early in the course of the disease before they begin to lose weight. Leptin levels fall in response to caloric restriction and negative energy balance. Women with amenorrhea and anorexia nervosa have lower levels of leptin than thin (BMI <18 kg/m2) women with normal menstrual cycles who have lower levels than normal-weighted women with normal cycles (50).
Inadequate nutrition from AN may impact growth velocity and final height, especially if the onset of weight loss occurs
before the pubertal growth spurt, if malnutrition is prolonged, and if IGF-1 is suppressed. The acceleration of growth after recovery may or may not allow for catch-up growth sufficient for attaining a normal final height (51,52,53,54). However, girls with AN often have a delayed bone age; in addition, if the onset of AN is after the growth spurt, height may be preserved.
before the pubertal growth spurt, if malnutrition is prolonged, and if IGF-1 is suppressed. The acceleration of growth after recovery may or may not allow for catch-up growth sufficient for attaining a normal final height (51,52,53,54). However, girls with AN often have a delayed bone age; in addition, if the onset of AN is after the growth spurt, height may be preserved.
Over the years, various methods have been proposed to ascertain the height, weight, percentage body fat, resting energy expenditure, and BMI associated with the restoration of menstrual cycles in girls with AN, sports-related weight loss, and chronic illness. Regardless of the method chosen, individual patients show variation in the weight at menarche as well as variation in weight at the recovery of menstrual function. In addition, obese patients may have amenorrhea accompanying weight loss although their weights remain above normal.
Forty years ago, Frisch and McArthur (55,56) examined the relationships between height/weight and menstrual function. Several methodologies have subsequently been used in determining what might be called the “treatment goal weight” for girls with weight loss or AN. The weight expected at the return of menses, however, also needs to take into account age, maturation, height, preillness weight, prior growth pattern, and growth potential (skeletal age). Although frequently the phrase “ideal body weight” (IBW) is used, the weight associated with optimal longevity has not been established in adolescents and with pubertal growth the weight would change. Thus, the preferred target is the “median body weight” (MBW) for height using percentiles for weight and height and the National Center for Health Statistics (NCHS) datasets (57). “Ideal” BMI (50th percentile of BMI for age) can also be obtained from Centers for Disease Control and Prevention (CDC) growth charts, the patient’s percentage of “ideal BMI” calculated (patient’s BMI divided by median BMI for age).
In studies of girls recovering from anorexia nervosa, 64% to 86% of girls with weight restoration have return of menses (58,59,60,61,62). Kreipe and colleagues (58,59) found that a mean of 92% ± 7% of IBW was associated with the return of menses. In another study, Golden and colleagues (60) noted that menses resumed at a weight 2.05 kg more than the weight at which menses were lost; the mean percent of standard body weight (SBW) was 91.6% ± 9.1% (Fig. 9-5). Eighty-six percent of patients resumed menses within 6 months of achieving this weight. Golden and colleagues (63) found that the mean BMI percentile associated with return of menses was 27.1 (95% confidence interval [CI]: 20.0 to 34.2) in a 1-year follow-up of 56 adolescents (64% resumed menses and 36% remained amenorrheic). Thus, patients benefit from being given a range of weights (92% to 100% MBW) and BMI (20th to 35th percentile), not a single number.
Some girls with AN remain amenorrheic despite weight restoration. Patients who regain a normal weight for height but persist with amenorrhea frequently continue to have abnormal eating patterns, avoidance of foods containing fat, and preoccupation with food. In a study of young women with persistent amenorrhea despite weight recovery, those with amenorrhea had similar BMI but higher scores on the Eating Disorder Inventory, lower leptin levels, and lower estradiol concentrations than normally cycling women (64). In a retrospective chart review of 382 normal-weight adolescents with a history of eating disorders, Sterling and colleagues found that the amenorrheic group had lower mean body weight, lower percent of ideal body weight, and lower mean resting energy expenditure than those with regular menses, suggesting an adaptation to the lower caloric and lower fat intake (65).
Female Athlete Triad: Disordered Eating, Amenorrhea, and Osteoporosis OR Low Energy Availability, amenorrhea, and Low Bone Mass
Over the past three decades, there has been an 800% increase in participation by adolescent girls in high school sports (66). Exercise clearly has benefits, including improved cardiovascular fitness, socialization, involvement in peer groups, a sense of well-being, weight control, lowering of blood pressure, and improved lipid profile. A lower rate of cancers of the reproductive system and breast cancer has been reported in one study of former athletes (67). However, intense exercise can be associated with amenorrhea and stress fractures. In the early 1990s, clinicians began to recognize the interrelatedness of disordered eating, amenorrhea, and osteoporosis in athletes (68). With more understanding of the pathophysiology, the “disordered eating” component of the triad has been replaced by the term “low energy availability (with or without an eating disorder)” (69). In addition, the terms osteopenia and osteoporosis are no longer used for children and adolescents, but reserved for postmenopausal women. In teens, calculation of the BMD Z-score (standard
deviations below sex- and age-matched controls) and use of the term “low bone mass below the expected range for age” for those with BMD Z-scores below -2.0 is preferred. The American College of Sports Medicine has suggested that because athletes usually have 5% to 15% higher BMD than nonathletes, the term “low BMD” should be used if the BMD Z-score is between -1.0 and -2.0 and there is a history of nutritional deficiency, hypoestrogenism, stress fractures, and/or other clinical risk factors for fractures (69).
deviations below sex- and age-matched controls) and use of the term “low bone mass below the expected range for age” for those with BMD Z-scores below -2.0 is preferred. The American College of Sports Medicine has suggested that because athletes usually have 5% to 15% higher BMD than nonathletes, the term “low BMD” should be used if the BMD Z-score is between -1.0 and -2.0 and there is a history of nutritional deficiency, hypoestrogenism, stress fractures, and/or other clinical risk factors for fractures (69).
The triad was originally noted in those girls who participated in sports in which achieving or maintaining an ideal body weight or optimal percentage of body fat was important and scoring of success was partly subjective (67,68). Coaching strategies such as daily weigh-ins and strict weight standards may predispose young women to the triad. A highly structured life, social isolation, lack of a support system, and a family history of disordered eating have also been associated with this presentation. However, a study of 170 athletes in 6 high schools in southern California found that 18% met the criteria for disordered eating, 23.5% for menstrual irregularity, and 21.8% for low bone mass, but few met all three criteria (70).
Depending on the sport, level of competition, age, and definition of amenorrhea, the incidence of amenorrhea in athletes has been reported at 3% to 66%, compared to an expected incidence of 2% to 5% (66,67,68,69,70,71,72,73,74,75,76,77,78) (Table 9-3). Gymnastics, ballet, and running are sports particularly associated with menstrual problems. Even swimmers (who have more body fat than runners on average) may experience menstrual dysfunction, most commonly short luteal phases. Although a 1980s study of adolescents involved in after-school sports programs found little risk of disrupted menstrual cycles (79), nearly one-quarter of current high school athletes have some menstrual irregularity (70). Even athletes with seemingly regular menses may in fact have anovulatory cycles or inadequate luteal phases (80,81).
Delayed pubertal development and primary amenorrhea have been observed particularly in thin ballet dancers, gymnasts, and runners (75,76). Ballet dancers often have delayed thelarche and menarche with a normal age of adrenarche, irregular menses, high energy output, and decreased caloric intake with episodes of bingeing (82,83). The delay seen in runners may also stem in part from the preselection of girls who have a thin body type and familial late development and who also excel in athletic endeavors (84). Training before menarche has been associated with a delay of menarche—5 months for each year of training (71).
Table 9-3 Factors Associated with Irregular Menses in Athletes | |
|---|---|
|
The intensity of the exercise and the age of the athlete also appear to be contributing factors to amenorrhea. In most studies, a greater number of miles run per week is associated with a higher incidence of amenorrhea. In a survey of college runners, the incidence of amenorrhea was 20% for women running 20 miles per week and 43% for those running 60 to 80 miles per week (85). Runners who are young and nulliparous are more likely to experience irregular cycles than older, multiparous runners.
Diet may be suboptimal in many adolescent athletes. Diets low in calories, fat, and red meat and high in carotene as well as vegetarian diets have been associated with amenorrhea (69,73,86,88), and disordered eating behaviors may accompany these food selections. Protein needs for athletes may also be higher (1.2 to 1.6 g/kg/day) than for nonathletes (69). In an attempt to separate weight loss from strenuous exercise as causative factors, Bullen and colleagues (74) carried out a prospective study of menstrual cycles, assigning one group to weight maintenance and the other to weight loss. They found that exercise, especially if accompanied by weight loss, could reversibly disturb menstrual function. Those in the weight loss group experienced more delayed menses, and a higher percentage of patients had loss of the LH surge.
Fifteen percent to 62% of young female athletes (61,68,76) have disordered eating, and 25% and 35% of elite athletes have eating disorders (66). For example, ballet dancers have higher Eating Attitudes Test (EAT) scores, a measure of disordered eating, than nondancers (mean 22.9 vs. 4.1) (89




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree