Chapter 20
The Pain of Childbirth and Its Effect on the Mother and the Fetus
Peter H. Pan MD, James C. Eisenach MD
Chapter Outline
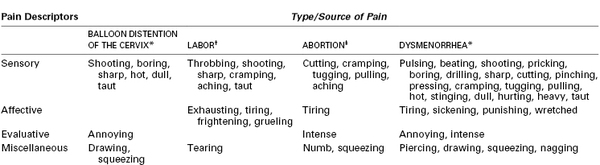
The gate control theory of pain, described more than 40 years ago by Melzack and Wall,1 has revolutionized the understanding of the mechanisms responsible for pain and analgesia. Originally explained as the regulation of pain signals from the peripheral nerve to the spinal cord by the activity of other peripheral nerves, interneurons in the spinal cord, and central supraspinal centers (Figure 20-1), the theory has been refined with the concept of a neuromatrix, a remarkably dynamic system capable of undergoing rapid change.2 Neural circuits and intraneural mechanisms regulate sensitivity at peripheral afferent terminals; along the conducting axons of peripheral nerves; in the spinal cord, pons, medulla, and thalamus; and at cortical sites of pain transmission and projection. For example, the peripheral application of capsaicin to the skin alters spinal gating mechanisms within 10 minutes, resulting in a light touch signal’s being interpreted as burning pain.3
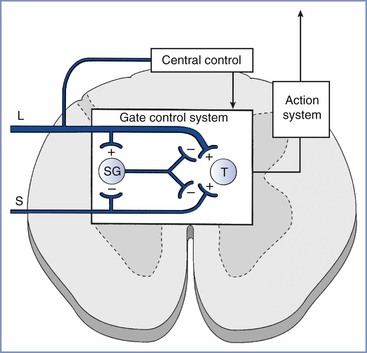
FIGURE 20-1 Gate control theory of pain. Activity in small-diameter afferents (S) stimulates transmission cells in the spinal cord (T ), which send signals supraspinally and results in the perception of pain. Small-diameter afferents also inhibit cells in the spinal cord substantia gelatinosa (SG), the activity of which reduces excitatory input to T cells. Activity in large-diameter afferents (L) also stimulates T cells in a manner that is perceived as nonpainful and excites SG cells to “close the gate” and reduce small-diameter afferent activation of T cells. The gate mechanism is under regulation by central sites. (From Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965; 150:971-5.)
Despite extensive research (initiated by the gate control theory) into the mechanisms and treatments for chronic pain, virtually no research on the neurophysiologic basis or therapies for labor pain has been performed. This discrepancy in focus has led to vastly different approaches to the treatment of patients with chronic versus obstetric pain. A patient with chronic pain typically undergoes a sophisticated physical assessment of sensory function; is offered therapies, on the basis of the assessment, from nearly a dozen different classes of analgesics; and can benefit from the enormous resources expended by the pharmaceutical industry to introduce agents that act on novel receptors or enzymes. By contrast, a laboring woman receives no physical assessment of sensory function and is offered only a handful of systemic drugs that act primarily through the anatomic blockade of neural traffic.
In this chapter, this paradox in the approach to labor pain is examined and the basis for current therapy (anatomy), the basis for future therapy (neurophysiology), and the effects of labor pain on the mother and the infant are reviewed.
Measurement and Severity of LABOR Pain
The recognition and acceptance of chronic pain, which frequently lacks an obvious outward cause, contrasts to the recurrent denial of labor pain, which is accompanied by visible tissue injury. Dick-Read4 suggested that labor is a natural process not considered painful by women in primitive cultures that should be handled with education and preparation rather than through pain medications. Lamaze5 popularized psychoprophylaxis as a method of birth preparation; this method now forms the basis for prepared childbirth training in the developed world. Although childbirth training acknowledges the existence of pain during labor, some scientific-thought leaders still consider labor pain to be minor.
The severity of labor pain has been recognized previously. Melzack,6 using a questionnaire developed to assess the intensity and emotional impact of pain, observed that nulliparous women with no prepared childbirth training rated labor pain to be as painful as a digit amputation without anesthesia (Figure 20-2).6 More than 30 years before Melzack’s quantification of pain, Javert and Hardy7,8 trained subjects to reproduce the intensity of labor pain with the sensation of noxious heat applied to the skin from a radiant heat source. In these experiments, several women achieved “ceiling pain”—resulting in second-degree burns to the skin—when they attempted to match the intensity of uterine contraction pain.7 Individual women also reported a close positive correlation between cervical dilation and pain intensity. Logistic regression analysis of the investigators’ original data7 indicates a high likelihood of severe pain as labor progresses, with a time course closely associated with cervical dilation (Figure 20-3). Other investigators have noted that uterine pressure during contractions accounts for more than 90% of the variability in labor pain intensity.9 These observations are consistent with the conclusion that cervical distention is the primary cause of pain during the first stage of labor.
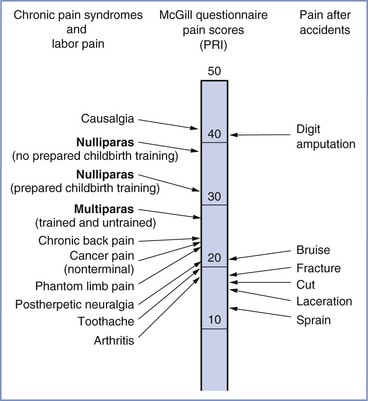
FIGURE 20-2 A comparison of pain scores obtained through the McGill Pain Questionnaire. Scores were collected from women in labor, patients in a general hospital clinic, and patients in the emergency department after accidents involving traumatic injury. Note the modest difference in pain scores between nulliparous women with and without prepared childbirth training. PRI, Pain rating index, which represents the sum of the rank values of all the words chosen from 20 sets of pain descriptors. (Modified from Melzack R. The myth of painless childbirth [The John J. Bonica Lecture]. Pain 1984; 19:321-37.)
FIGURE 20-3 Likelihood of severe pain during labor. A significant minority of women (approximately one third) have severe pain in early labor, and the proportion of women with severe pain increases to nearly 90% later in labor, in close relationship with cervical dilation. (Data adapted from Hardy JD, Javert CT. Studies on pain: measurements of pain intensity in childbirth. J Clin Invest 1949; 28:153-62.)
There is considerable variability in the rated intensity of pain during labor. Nulliparous women rate labor pain as more severe than parous women; however, the differences are small and of questionable clinical relevance.10 There is a correlation between the intensity of menses and labor pain, especially back discomfort,10 although the reason for this relationship is unknown. It is possible that the rated intensity of labor pain reflects individual differences in the perception of all types of pain. In a study of factors affecting labor pain, 10 of 97 subjects reported that they had never experienced pain before childbirth; these women reported significantly less pain during labor and delivery compared with women who had previously experienced pain.11 In other studies, the variability of pain after cesarean delivery could be predicted with preoperative quantitative sensory testing (such as rating the intensity of pain with a standardized noxious thermal stimulus), psychologic constructs, and their combinations.12,13
The mechanism by which people perceive different levels of pain from the same stimulus remains unclear. A study involving brain imaging and a fixed acute noxious heat stimulus showed a strong correlation between verbal pain assessment and the level of activation of various cortical brain regions, especially the contralateral somatosensory cortex and anterior cingulate cortex.14 The investigators also found that the degree of activation of the thalamus was essentially identical in all subjects, suggesting that differences in perceived pain resulted from modulation at suprathalamic levels rather than in the peripheral nerves or spinal cord. The situation in labor may be more complex. For example, a large genetic polymorphism regulates cytokine production and function as well as pregnancy outcome.15 It is possible that interindividual differences in labor pain may partially reflect genetic differences in cytokine production or response.
In evaluating and studying labor pain and its treatment, most studies have tended to assess labor pain by using a set of discrete pain scores. However, labor pain is a complex, subjective, multidimensional, and dynamically changing experience with both sensory and affective components that are influenced by many factors. As a result, there are substantial individual differences in labor pain. Therefore, better identification of the covariates that affect labor progress and its associated pain is needed. Recently, Conell-Price et al.16 developed and validated a dynamic model to account for labor progress in the assessment of labor pain. Subsequently, Debiec et al.,17 at the same institution, combined a biexponential model that describes labor progress with a sigmoidal labor pain model to assess the influence of patient covariates on labor pain.17 Both studies used retrospective patient data to develop and test their models. In the former study,16 the prediction error for the pain scores was large, but the purpose of the model was to identify and remove variability associated with labor progress so that other factors (e.g., genetic polymorphisms) can be quantitatively studied. In this study,16 cervical dilation accounted for only 16% to 20% of the variability in reported pain. In the latter study,17 the covariate of ethnicity was found to have a statistically significant but clinically trivial effect on labor progress. The modeling described by these investigators provides a useful quantitative tool for future studies to identify and assess the effect—or the lack of effect—of patient and/or environmental covariates on labor progress, labor pain, and therapeutic responses. Better understanding of underlying causes of interindividual variability in labor progress, labor pain, and therapeutic responses is likely to lead to more tailored therapy.
In summary, although significant variability exists in the rated intensity of pain during labor and delivery, the majority of women experience more than minimal pain. The close correlation between cervical dilation and the rated severity of pain implies the existence of a causal relationship and increases the likelihood that a parturient will request analgesia as labor progresses.
Personal Significance and Meaning
The International Association for the Study of Pain (IASP) has defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”18 Clearly, this reflects an intensity-discriminatory component and an emotional-cognitive component, with powerful interactions between the two. The focus of current interventions is heavily weighted toward the first component and assumes that labor pain is severe and in need of pharmacologic treatment. Largely ignored are coping strategies and the personal meaning of labor pain, which varies considerably among women.19
Although many women rate the pain of labor and delivery as severe, the terms used to more fully describe this pain reflect an emotional meaning. In a pioneering study of the quantification of pain from experimental dilation of the cervix, Bajaj et al.20 compared pain descriptors in women who were in labor, had experimental cervical dilation, were undergoing spontaneous abortion, or who had dysmenorrhea (Table 20-1). Women with dysmenorrhea used words that indicate suffering, such as “punishing” and “wretched,” whereas those in labor did not. Some researchers have drawn parallels between the pain derived from mountain climbing, which is associated with a sense of euphoria, and the pain of labor.19 As noted by one woman, “You mature and become a stronger personality when you’ve had a baby and have gone through the pain. I think that is the purpose of it, what the meaning of life is … to protect our children, to be stronger.”21 However, other women have found no deeper meaning to the pain of labor or reasons why it should not be treated. Many conditions that involve pain (e.g., trauma, severe dental disease, cancer) are considered a “normal” part of human life without a spiritual meaning, thereby making labor pain unique.
In summary, there are large interindividual differences in how women experience the personal significance or meaning of labor pain. These different perceptions can lead to a long-term sense of failure and guilt when pharmacologic pain relief is accepted or emotional trauma when it is withheld.
Anatomic Basis
First Stage of Labor
Several lines of evidence suggest that the pain experienced during the first stage of labor is transduced by afferents with peripheral terminals in the cervix and lower uterine segment rather than the uterine body, as is often depicted (Figure 20-4). Uterine body afferents fire in response to distention, but in the absence of inflammation, uterine body distention has no or minimal effect on the behavior of laboratory animals.22,23 These observations suggest that uterine body afferents may be an important site of chronic inflammatory disease and chronic pelvic pain but are much less relevant to acute obstetric and uterine cervical pain. In addition, afferents to the uterine body regress during normal pregnancy, whereas those to the cervix and lower uterine segment do not.24 This denervation of the myometrium may protect against preterm labor by limiting α1-adrenergic receptor stimulation by locally released norepinephrine. Hardy and Javert8 reproduced the pain of uterine contractions in women during labor by manual distention of the cervix. Bonica and Chadwick25 later confirmed that women undergoing cesarean delivery under a local anesthetic field block experience pain from cervical distention (which mimics that of labor pain) but do not experience pain from uterine distention.25
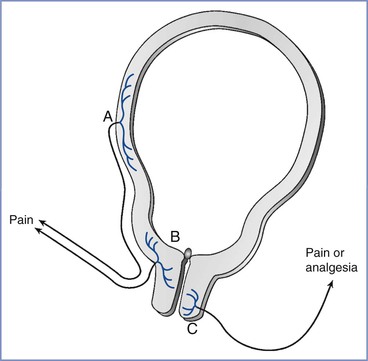
FIGURE 20-4 Uterocervical afferents activated during the first stage of labor. Uterine body afferents (A) partially regress during pregnancy and may contribute to the pain of the first stage of labor. However, the major input is from afferents in the lower uterine segment and endocervix (B). By contrast, at least in animals, the activation of afferents that innervate the vaginal surface of the cervix (C) result in analgesia, not pain, and they enter the spinal cord in sacral areas rather than at the site of referred pain in labor.
The uterine cervix has dual innervation; afferents innervating the endocervix and lower uterine segment have cell bodies in thoracolumbar dorsal root ganglia (DRG), whereas afferents innervating the vaginal surface of the cervix and upper vagina have cell bodies in sacral DRG.26 These two innervations result in different sensory input and referral of pain. Pelvic afferents that innervate the vaginal surface of the cervix are almost exclusively C fibers, with the majority containing the peptides substance P and calcitonin gene–related peptide (CGRP). These afferents express alpha and beta estrogen receptors and have an innervation pattern that is not affected by pregnancy.27–29 Stimulation of the vaginal surface of the cervix in rats results in antinociception, lordosis, ovulation, and a hormonal state of pseudopregnancy, all of which are related to mating behaviors in this species.30 In rats, these vaginal afferent terminals are activated only during delivery and not during labor, which suggests that they are not relevant to the pain of the first stage of labor.31 By contrast, dilation of the endocervix in rats results in the activation of afferents entering the lower thoracic spinal cord and nociception rather than antinociception. These afferents, which are mostly or exclusively C fibers,32 are activated during the first stage of labor, suggesting that they are relevant to pain during this period.
More than 80 years ago, experiments in dogs allowed Cleland33 to identify T11 to T12 as the segmental level of entry into the spinal cord of afferents that transmit the pain of the first stage of labor. Because dysmenorrhea could be treated through the destruction of the superior or inferior hypogastric plexus,34 Cleland reasoned that the sensory afferents and sympathetic efferents were likely intermingled; he subsequently demonstrated that the bilateral blockade of the lumbar paravertebral sympathetic chain could produce analgesia during the first stage of labor.33 First-stage labor pain is transmitted by afferents that have cell bodies in T10 to L1 DRG and pass through the paracervical region, the hypogastric nerve and plexus, and the lumbar sympathetic chain.
Classical teaching states that pain-transmitting C and A-delta nerve fibers enter the spinal cord through the dorsal roots and terminate in a dense network of synapses in the ipsilateral superficial laminae (I and II) of the dorsal horn, with minimal rostrocaudal extension of fibers. Whereas this characterization is true for somatic afferents, visceral C fiber afferents enter the cord primarily—but not exclusively—through the dorsal roots and terminate in a loose network of synapses in the superficial and deep dorsal horn and the ventral horn. These afferents also cross to the contralateral dorsal horn, with extensive rostrocaudal extension of fibers. This anatomic distinction underlies the precise localization of somatic pain and the diffuse localization of visceral pain, which may cross the midline; it may also determine the potency or efficacy of drugs that must reach afferent terminals, such as intrathecal opioids.
Pain-transmitting neurons in the spinal cord dorsal horn send axons to the contralateral ventral spinothalamic tract (stimulating thalamic neurons) with further projections to the somatosensory cortex, where pain is perceived. These spinal neurons also send axons through the spinoreticular and spinomesencephalic tracts to provide signals to the areas of vigilance (locus coeruleus, reticular formation), cardiorespiratory regulation (nucleus tractus solitarius, caudal medulla), and reflex descending inhibition (periaqueductal gray, locus coeruleus and subcoeruleus, nucleus raphe magnus, rostral medial medulla, cerebellum). Thalamic activation from painful stimuli results in the activation not only of the somatosensory cortex but also areas of memory (prefrontal cortex), motor response (M1 motor cortex), and emotional response (insular cortex, anterior cingulate cortex). Supraspinal pain pathways activated by pain of the first stage of labor can be briefly described sequentially, starting with the ascending pathways projecting to the pons and the medulla, thereby activating centers of cardiorespiratory control and descending pathways as well as the thalamus, which in turn sends projections to the anterior cingulate, motor, somatosensory, and limbic regions.
The anatomic basis for pain of the first stage of labor implies that amelioration of pain should occur after blockade of peripheral afferents (by paracervical, paravertebral, lumbar sympathetic, or epidural [T10 to L1 dermatome] block) or after blockade of spinal cord transmission (by intrathecal injection of local anesthetic and/or opioid) (Figure 20-5). In addition, the widespread distribution of visceral synapses in the spinal cord implies that intrathecally administered drugs (e.g., opioids) must have physicochemical properties that facilitate deep penetration into the cord to reach the terminals responsible for pain transmission.
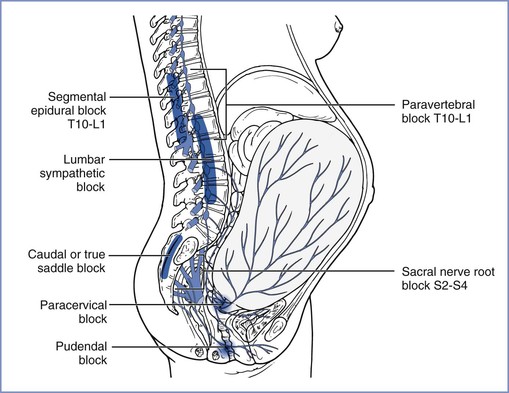
FIGURE 20-5 Transmission of labor pain. Labor pain has a visceral component and a somatic component. Uterine contractions may result in myometrial ischemia, which causes the release of potassium, bradykinin, histamine, and serotonin. In addition, stretching and distention of the lower segments of the uterus and the cervix stimulate mechanoreceptors. These noxious impulses follow the sensory nerve fibers accompanying sympathetic nerve endings, travel through the paracervical region and the pelvic and hypogastric plexus, and enter the lumbar sympathetic chain. Through the white rami communicantes of the T10, T11, T12, and L1 spinal nerves, they enter the dorsal horn of the spinal cord. These pathways could be mapped successfully by demonstration that blockade at different levels along this path (sacral nerve root block of S2-4, pudendal block, paracervical block, low caudal or true saddle block, lumbar sympathetic block, segmental epidural block of T10-L1, and paravertebral block T10-L1) can alleviate the visceral component of labor pain. (From Eltzschig HK, Lieberman ES, Camann WR. Regional anesthesia and analgesia for labor and delivery. N Engl J Med 2003; 348:319-32.)
Second Stage of Labor
Pain during the second stage of labor is transmitted by the same afferents activated during the first stage of labor but with additional afferents that innervate the cervix (vaginal surface), vagina, and perineum. These additional afferents course through the pudendal nerve DRG at S2 to S4 and are somatic. Thus, the pain specific to the second stage of labor is precisely localized to the vagina and perineum and reflects distention, ischemia, and frank injury, either by stretching to the point of disruption or by surgical incision. Studies in nonpregnant women indicate a minor analgesic effect of mechanical self-stimulation of the vaginal surface of the cervix35; this effect may result from the stimulation of C fibers, because in women with a high oral intake of capsaicin, the activity of such fibers is reduced.36 The relevance of this minor effect in reducing the pain of the second stage of labor is questionable and has not been examined; however, it does appear to provide evidence that noxious input during labor may activate endogenous analgesia (see later discussion).
The anatomic basis for pain of the second stage of labor implies that analgesia can be obtained through a combination of methods used to treat the pain of the first stage with a pudendal nerve block or extension of the epidural blockade from T10 to S4 (see Figure 20-5).
Neurophysiologic Basis
Peripheral Afferent Terminals
Visceral nociceptors, such as those that transduce the pain of the first stage of labor, are activated by stretching and distention. However, unlike somatic afferents, they are not activated by cutting. With each uterine contraction, pressure is transmitted to distort and stretch the uterine cervix, thereby leading to the activation of these nerve terminals. How mechanical distention results in the depolarization of the nerve terminal and the generation of an action potential is not entirely known, but the following three mechanisms are likely:
1. A variety of ion channels respond to the distortion of the cell membrane, and one of them—brain sodium channel-1 (BNC-1) or acid-sensing ion channel-2 (ASIC-2)—is exclusively expressed in sensory afferents and might directly depolarize the nerve terminal by opening its channel when the membrane is distorted (Figure 20-6).37
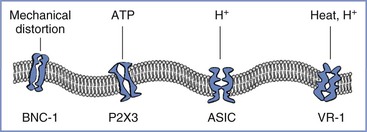
FIGURE 20-6 Afferent nerve endings contain multiple excitatory ligand-gated ion channels, including those that respond to mechanical distortion: BNC-1, brain sodium channel-1; ATP, adenosine triphosphate; P2X3, purinergic receptor; H +, hydrogen ion; ASIC, acid-sensing ion channel; VR-1, vanilloid receptor type 1.
2. Mechanical distortion may result in the acute release of a short-acting neurotransmitter that directly but transiently stimulates ion channel receptors on nerve terminals. Although this process has not yet been examined in the uterine cervix, studies have observed that stretching the bladder urothelium releases adenosine triphosphate, which directly stimulates a type of ligand-gated ion channel—P2X3—on sensory afferents in the bladder wall.38 Because P2X3 receptors are widely expressed in C fibers,39 this mechanism might be responsible for the pain that results from the acute distention of the uterine cervix.
3. Local ischemia during contractions may result in gated or spontaneous activity of other ion channels. Some of these ion channels—the ASIC family—respond directly to the low pH that occurs during ischemia,40 whereas other classes of ion channels may be activated to open spontaneously. For example, the vanilloid receptor type 1 (VR-1) can be stimulated by capsaicin. It is likely that VR-1 receptors (which also respond to noxious heat) are expressed on visceral afferent terminals, given that the application of capsaicin or heat to the distal esophagus in humans results in pain.41 VR-1 receptor–gated ion channels are not normally open in the absence of high temperature or capsaicin-like ligands; however, in the presence of low pH, the temperature response of these receptors shifts so that their channels open at body temperature.42
Uterine cervical afferents (including the C fibers that innervate the vaginal surface of the cervix) contain substance P, CGRP, and the enzyme nitric oxide synthase.43 C fibers can be divided into two groups: (1) those that contain substance P and CGRP and respond to nerve growth factor through actions on tyrosine kinase A receptors and (2) those that contain somatostatin, instead of substance P and CGRP, and respond to glially derived growth factor through actions on a c-ret complex.44 Some overlap exists between these rough classifications, and further definition of C fiber subtypes will likely occur as more markers and neuropeptides are examined. Other compounds commonly contained in C fiber terminals include glutamate, vasoactive intestinal peptide, and neuropeptide Y. The variable role of C fiber subtypes in the transmission of pain is also unclear. Given that somatostatin typically inhibits substance P release and pain transmission,45,46 the net transmission of nociception at the spinal cord level may reflect a complex interaction between excitatory and inhibitory C fiber subtypes.
The peripheral afferent neurophysiology of pain during the first stage of labor suggests that the largely unexplored multiple ion channels that transduce the mechanical signal of cervical stretching to an electrical signal generating the perception of pain may represent important new targets for local or systemic analgesic drug delivery. In addition, the understanding of the classification, function, and relevance to pain of different C fiber subtypes remains in its infancy. Research involving endocervical C fiber subtypes may identify new targets for the treatment of labor pain.
Role of Sensitization
Peripheral afferent terminals, like other parts of the sensory system, can change their properties in response to various conditions. Afferent terminals can be directly stimulated by the low pH associated with inflammation (Figure 20-7), and selective ligand-gated ion channels on these terminals can be stimulated by the release of bradykinin.47 In addition, peripheral inflammation sensitizes afferent terminals by changing their properties; this process can result, over a short time, in a change in gene expression by these nerve fibers, thereby leading to a large amplification of pain signaling.
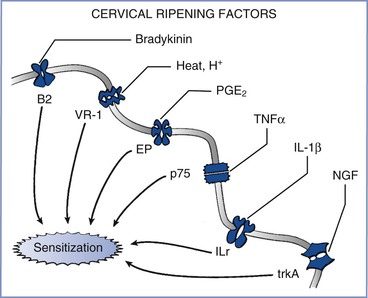
FIGURE 20-7 Effects of inflammation from cervical ripening on afferent terminals. A variety of factors—including bradykinin, heat and hydrogen ions, prostaglandins (including PGE2), tumor necrosis factor-α (TNFα), interleukin-1 beta (IL-1β), and nerve growth factor (NGF)—act on their cognate receptors to sensitize nerve endings and amplify the perception and severity of pain from nerve stimulation. B2, bradykinin-2 receptor; EP, prostaglandin E receptor; ILr, interleukin-1 receptor; p75, p75 tumor necrosis factor-α receptor; trkA, tyrosine kinase A; VR-1, vanilloid receptor type 1.
Although peripheral inflammation is most commonly associated with the pain that results from acute postoperative and chronic arthritic conditions, it may also play an essential role in labor pain. The cervical ripening process and labor itself both result from local synthesis and release of a variety of inflammatory products. The clinical implications of these inflammatory pathways include the application of inflammatory mediators (e.g., prostaglandin E2 [PGE2]) to prepare the cervix for labor induction and the administration of inflammatory mediator inhibitors (e.g., indomethacin) to stop preterm labor.
PGE2 is an especially important sensitizing agent for uterine cervical afferents. In most species, the onset of labor is triggered by a sudden decrease in circulating estrogen concentration. This decrease removes a tonic block on the expression of cyclooxygenase, leading to an increase in local production of prostaglandins, especially PGE2.48 PGE2 is central to a variety of processes that are activated to allow ripening and dilation of the uterine cervix. During the 24 to 72 hours preceding the onset of labor, collagen in the cervix becomes disorganized owing to the activation of prostaglandin receptors and the activity of inflammatory cytokines (mostly interleukin-1-beta [IL-1β] and tumor necrosis factor-alpha [TNF-α]) and matrix metalloproteinases (especially types 2 and 9).49,50 A series of studies in the rat paw have demonstrated that PGE2 induces peripheral sensitization in a sex-independent manner by activation of protein kinase A51 and nitric oxide synthase.52
Cytokines and growth factors are also released into the uterine cervix immediately before and during labor. The cytokine IL-1β enhances cyclooxygenase activity and substance P release in the DRG and spinal cord.53,54 TNF-α increases the spontaneous activity of afferent fibers55 and enhances CGRP release and VR-1 receptor expression in DRG cells in culture.56 Nerve growth factor also induces mechanical hypersensitivity.57 These sensitizing substances (prostaglandins, cytokines, and growth factors) signal peripheral nerves in a manner that results in a host of changes in DRG cell number, peptide expression and release, receptor and ion channel expression, and biophysical properties. For example, inflammatory mediators alter the expression of sodium (Na+) channel subtypes,58,59 thereby resulting in more rapid, repetitive firing capability60 and spontaneous afferent activity.61
Estrogen receptor signaling can dramatically affect the structure of the uterine cervix and possibly modulate pain responses. Long-term estrogen exposure sensitizes a subset of mechanosensitive afferents innervating the uterine cervix. The hypogastric afferents that innervate the uterine cervix are polymodal and contain high-threshold (HT) and low-threshold (LT) fibers. Long-term estrogen exposure increases the spontaneous activities of both HT and LT fibers, but only HT fibers show greater responses to uterine cervical distention.62 Long-term estrogen exposure also increases the proportion of hypogastric afferents innervating the uterine cervix, which express transient receptor potential vanilloid type 1 (TRPV-1). Capsazepine, a TRPV-1 channel antagonist, reduces the hypogastric afferent responses to cervical distention in estrogen-treated animals but not in ovariectomized animals without estrogen replacement.63,64
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree