Chapter 15
In Vitro Fertilization and Other Assisted Reproductive Technology
Lawrence C. Tsen MD
Chapter Outline
ASSISTED REPRODUCTIVE TECHNOLOGY PROCEDURES
SUCCESS OF ASSISTED REPRODUCTIVE TECHNOLOGY
EFFECTS OF ANESTHESIA ON REPRODUCTION
In 1978, Steptoe and Edwards1 reported the first live birth of an infant produced from in vitro fertilization (IVF) techniques. Their case highlighted the laparoscopic recovery of a single oocyte just before ovulation in a natural menstrual cycle; after in vitro insemination, the resulting embryo was grown in culture media for 2.5 days to the eight-cell stage and was transferred to the uterine cavity (i.e., embryo transfer [ET]).
IVF techniques were initially developed as a treatment for infertility secondary to chronic fallopian tube disease. Current indications for this emerging spectrum of new techniques, which as a group are referred to as assisted reproductive technology (ART), include (1) inadequate oocyte quality or number (donor oocyte therapy), irreparability or absence of the uterus (surrogate uterus programs), and significant co-morbidities (embryo and ovarian tissue cryopreservation) in women; (2) sperm deficiencies in men; and (3) certain genetic aberrations in couples.2
In 1981, Edwards3 estimated that 15 to 20 infants would be born worldwide through the use of IVF and ET techniques. Advances in the science and international acceptance of ART procedures have resulted in a dramatic increase in the number of infants born globally (Figure 15-1).4 A similar increase in the use of these technologies has been witnessed in the United States (Figure 15-2); in 2011, the initiation of 163,038 ART cycles resulted in the birth of 61,610 infants.5
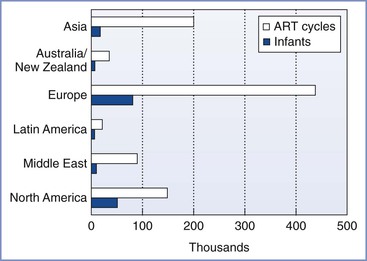
FIGURE 15-1 Numbers of initiated assisted reproductive technology (ART) cycles and infants born worldwide by region in 2011 (from data accumulated in 2003 from 54 countries) as reported by the International Committee for Monitoring Assisted Reproductive Technology (ICMART). (Data from Nygren KG, Sullivan E, Zegers-Hochschild F, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) world report. Assisted Reproductive Technology 2003. Fertil Steril 2011; 95:2209-22, 22.e1-17.)
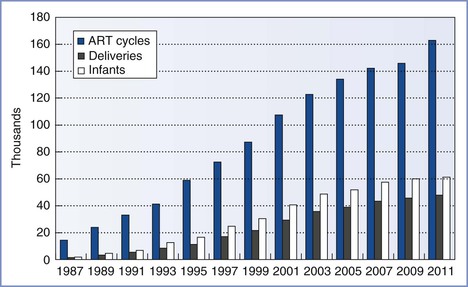
FIGURE 15-2 Numbers of assisted reproductive technology (ART) cycles performed, live-birth deliveries, and infants born in the United States using ART from 1987 through 2011, as reported to the Centers for Disease Control and Prevention, Division of Reproductive Health, and the Society for Assisted Reproductive Technology Registry. (Data from U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Division of Reproductive Health. 2011 Assisted Reproductive Technology [ART] Report. Atlanta, CDC/DRH, 2013.)
Despite the application of ART procedures to more diverse and challenging causes of infertility, the probability of a live birth after a cycle of hormonal stimulation has increased from 6% in 1985 to 30% in 2011.5 Attention to subtle differences in culture media as well as improvements in laboratory methods, retrieval routes, and transfer techniques are primarily responsible for these improved results.6 Given the importance of small alterations to the overall success of ART, coupled with the associated costs (i.e., approximately $10,000 for each cycle that progresses to transfer and limited insurance coverage for these procedures),7 it is prudent for anesthesia providers to be aware of the potential effects that anesthetic agents may have on gametes or embryos.
Assisted Reproductive Technology Procedures
Hormonal Stimulation
Although the success of IVF was initially limited by the single preovulatory oocyte generated with each natural menstrual cycle,1 the introduction of follicular hormonal stimulation has significantly increased the probability of a live birth through the retrieval of multiple oocytes per cycle. Hormonal regimens typically initiate a cycle with gonadotropin-releasing hormone agonist to induce pituitary and ovarian suppression, followed by follicle-stimulating hormone and human menopausal gonadotropin to stimulate the development and growth of multiple ovarian follicles. Human chorionic gonadotropin (hCG) is later added to induce maturation and demargination of the oocyte from the follicular wall before retrieval. Although the goal of these regimens is the generation of 10 to 15 oocytes, superovulation can occur, resulting in the production of as many as 70 oocytes. All visible ovarian follicles are aspirated (see later discussion), with each follicle usually containing a single oocyte.
After oocyte retrieval, pituitary function is usually insufficient to provide adequate hormonal support to the growing corpus luteum. For this reason, parenteral progesterone is often provided daily until either the results of the pregnancy test are known or the first trimester of pregnancy is completed.
Oocyte Retrieval
Originally conducted with direct visualization of the ovarian follicles through pelvic laparoscopy,1 the majority of oocyte retrievals are currently performed through a transvaginal approach with ultrasonographic guidance (Figure 15-3).8 Laparoscopic oocyte retrieval is typically reserved for situations in which tubal transfer is planned (i.e., gamete intrafallopian transfer [GIFT] or zygote intrafallopian transfer [ZIFT]; see later discussions).
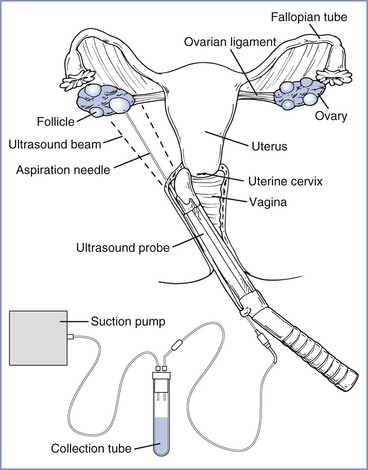
FIGURE 15-3 Transvaginal ultrasound-guided oocyte retrieval. The ultrasound probe is placed in the vagina and advanced into the posterior fornix. The needle, previously inserted through the needle guide, is advanced through the vaginal wall and ovarian capsule. (Redrawn from Steinbrook R. Egg donation and human embryonic stem-cell research. N Engl J Med 2006; 354:324-6. Copyright © 2006 Massachusetts Medical Society. All rights reserved.)
Oocyte retrieval is performed 34 to 36 hours after hCG administration. Retrieval must be performed promptly to prevent spontaneous ovulation from reducing the number of mature oocytes. With the use of a transvaginal ultrasound probe to visualize the ovary, mature follicles are punctured and aspirated with a needle introduced through the vaginal fornix. Oocytes are immediately washed in culture media and examined microscopically to determine their stage of meiosis. Oocytes are classified as postmature metaphase II, mature metaphase II, metaphase I, or prophase I on the basis of their nuclear, cytoplasmic, and extracellular composition.
In Vitro Fertilization
Although the term in vitro fertilization is often used synonymously with any aspect of ART, technically it applies only to the process of oocyte fertilization with spermatozoa in culture media. After a microscopic examination, oocytes are incubated for 4 to 6 hours in culture media that resembles human fallopian tube fluid and are then inseminated. The insemination process is sometimes delayed with immature oocytes (e.g., metaphase I) in an attempt to increase the probability of normal (i.e., monospermic) fertilization.
At 16 to 20 hours after insemination the oocytes are examined for evidence of fertilization (i.e., the presence of two pronuclei and two polar bodies in the perivitelline space) (Figure 15-4).9 The advantages of IVF include the ability to document the process of fertilization and to use techniques to improve sperm motility or penetration (e.g., intracytoplasmic sperm injection). IVF followed by ET represents approximately 99% of the ART procedures used in the United States5; less than 1% occurs via GIFT or ZIFT procedures (see later discussions). Male factor infertility is present in approximately 35% of the couples seeking ART procedures, and intracytoplasmic sperm injection is currently used in more than 65% of the cases treated annually in the United States.5
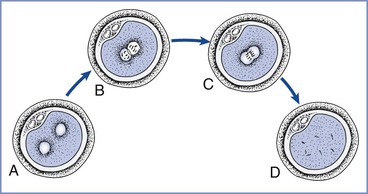
FIGURE 15-4 Pronuclear stage prezygote. A, At 8 to 10 hours after insemination, pronuclei are barely visible and may be spaced slightly apart. B, After 12 hours, pronuclei have migrated to the center of the cell and are clearly seen. C, At 20 to 22 hours, nuclear envelopes break down and pronuclei begin to fade from view. D, The one-cell zygote before the first cleavage. (Redrawn from Veeck LL. Atlas of the Human Oocyte and Early Conceptus. Baltimore, Williams & Wilkins, 1991:43.)
Embryo Transfer
Embryos resulting from IVF may be transferred into the fallopian tubes (i.e., ZIFT) or the uterine cavity (IVF-ET). Most ET procedures are performed transcervically 3 days after retrieval, with the embryos transferred via a catheter. The advantages of transcervical ET are (1) simplicity—it does not require laparoscopy or anesthesia; (2) low cost, especially compared with laparoscopic intrafallopian transfer procedures; and (3) the ability to proceed without patent fallopian tube(s). The primary disadvantage of transcervical ET is that the probability of successful pregnancy is slightly less than that with an ET performed directly into the fallopian tubes (i.e., ZIFT). Embryos in excess of those required for transfer may be frozen in 1,2-propanediol or glycerol and stored for possible later transfer.
Gamete Intrafallopian Transfer
GIFT procedures consist of the transabdominal or transvaginal collection of oocytes followed by a microscopic inspection of the oocytes’ quality and maturation in a laboratory adjacent to the operating room. Mature oocytes are aspirated into a transfer catheter with washed partner or donor sperm, and the contents (gametes) are injected into the distal 3 to 6 cm of one or both fallopian tubes. The catheter is subsequently inspected microscopically to verify that oocytes have not been retained. The GIFT procedure does not involve IVF, because fertilization occurs in vivo in the natural milieu of the fallopian tube.
Specific advantages of the GIFT procedure include (1) the convenience of oocyte retrieval and ET occurring within a single operative event, (2) the elimination of IVF, and (3) the fact that the embryos reach the uterine cavity at a potentially more appropriate (i.e., later) stage of development than with IVF-ET.10 The primary disadvantage is that fertilization cannot be documented; an issue that may be critical when this capacity is in question (e.g., couples with male or immunologic factors). Normally, 50% to 70% of inseminated oocytes become fertilized11; however, lower fertilization rates are often observed in couples with severe male factor infertility or in women with antisperm antibodies. Other limitations are the required presence of at least one patent fallopian tube and the requirement for laparoscopic surgery.
Zygote Intrafallopian Transfer
ZIFT (also known as pronuclear stage transfer [PROST]) consists of oocyte retrieval followed by IVF. At 16 to 20 hours after insemination the oocytes are examined for the presence of two distinct pronuclei (i.e., the pronuclear stage; see Figure 15-4), which indicates that fertilization has occurred. The patient is anesthetized for laparoscopy, and pronuclear stage embryos (usually no more than four) are transferred through a catheter into the distal portion of a fallopian tube (as described for GIFT). Advantages of ZIFT include (1) the documentation of fertilization, (2) the avoidance of laparoscopy if fertilization is not successful (approximately 13% of inseminations),5 (3) a shorter exposure to the laboratory environment than with IVF-ET, and (4) the potential for embryos to reach the uterine cavity at a more appropriate stage of development than with IVF-ET (i.e., approximately the fifth day after insemination). Its disadvantages and limitations include (1) the added inconvenience and cost of a two-stage procedure, (2) the requirement for laparoscopic surgery, and (3) the presence of at least one patent fallopian tube.
Success of Assisted Reproductive Technology
The Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) collaborate with the Centers for Disease Control and Prevention (CDC) to maintain a data registry and analyze the results of all ART cycles initiated during each calendar year in the United States.5
Maternal age is the dominant factor in determining the likelihood of a successful pregnancy after an ART procedure (Figure 15-5). For example, in 2010, 41.5% of IVF cycles in women younger than 35 years led to the delivery of one or more infants.5 By contrast, in a similar group of women older than 42 years who met optimal fertility criteria, only 6% of these procedures resulted in a live birth.5 In 2010, the average age of a woman having an ART procedure in the United States was 36 years.
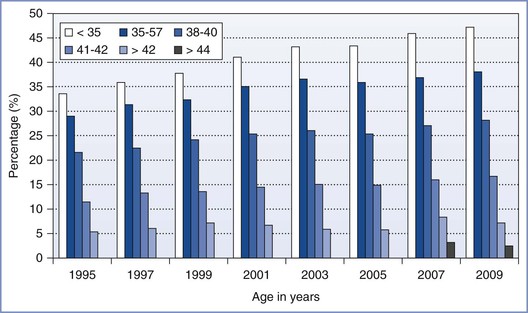
FIGURE 15-5 Percentage of transfers that resulted in live births with assisted reproductive technology (ART) cycles using fresh nondonor eggs or embryos, according to the women’s age in the years from 1995 to 2009, as reported to the Centers for Disease Control and Prevention, Division of Reproductive Health, and the Society for Assisted Reproductive Technology Registry. The first year in which data for women older than 42 were subdivided into ages 43 to 44 and older than 44 was in 2007. (Data from U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Division of Reproductive Health. 2009 Assisted Reproductive Technology (ART) Report. Atlanta, CDC/DRH, 2011.)
Although pregnancy and delivery rates have historically been greater for tubal transfers (i.e., GIFT, ZIFT) than for transcervical uterine transfers (IVF-ET), greater parity in these rates has developed in recent years.5,11 The early postovulatory uterine environment has been postulated to be unfavorable to early embryo growth.11 Tubal transfer procedures allow embryos 3 to 5 days to reach the uterine cavity, when the environment for implantation may be more receptive. Lower implantation rates after transcervical ET may also be explained by (1) adverse uterine effects produced by the transfer procedure, (2) uterine contractions expelling transfer fluid and embryos,12 and (3) the absence of yet undiscovered tubal factors that promote early embryo growth and implantation.11,13
Obstetric Complications
ART hormonal stimulation regimens are associated with increased coagulation and decreased fibrinolysis when evaluated by individual hemostatic markers and global assessment tools (i.e., thromboelastography).14,15 These alterations are especially significant in the setting of the most common ovarian stimulation complication, a phenomenon termed ovarian hyperstimulation syndrome (OHSS). Mild cases of OHSS may manifest as abdominal discomfort, bilateral ovarian enlargement, and ascites, whereas severe cases may result in follicular rupture and hemorrhage, pleural effusion, hemoconcentration, oliguria, and thromboembolic events.16–18 The anesthetic implications of OHSS include increased free drug concentrations (see later discussion) and greater perioperative pain from larger follicle numbers. The fullest expression of the syndrome usually occurs after oocyte retrieval, particularly if the decision is made to proceed with an ET, which maintains exposure to endogenous and exogenous hormones. Rarely, an emergency laparoscopy or laparotomy is required for excision of a ruptured ovarian cyst or the release of an ovarian pedicle torsion.18 Abdominal paracentesis and thoracentesis may be necessary before the induction of general anesthesia in patients with respiratory compromise due to massive ascites or pleural effusions.
Multiple-gestation pregnancies represented 31% of the deliveries that followed ART procedures in the United States in 2010, of which 91% were twins.5 The transfer of a greater number of embryos or oocytes increases both the probability of a live birth and the likelihood of a multifetal pregnancy. Although many infertile couples would prefer a twin or triplet pregnancy, maternal and perinatal morbidity and mortality for multiple versus singleton gestation pregnancies is at least doubled.19 In addition, overall medical costs escalate with each additional fetus, particularly if the birth occurs preterm. Within the United States in 2006, 62% of ART twins and 97% of ART triplets were delivered preterm, resulting in an estimated financial burden of $1 billion.20 In an effort to reduce the incidence and sequelae of multifetal pregnancies, many ART programs and societies, and even some countries, have mandated a limit on the number of embryos or oocytes that are transferred.21 In the presence of triplet or higher-order gestation pregnancies, selective reductions can be performed; however, these procedures are subject to a number of medical and ethical considerations.
Ectopic pregnancies occur up to five times more frequently in ART pregnancies than with natural pregnancies (2%), primarily owing to the greater prevalence of fallopian tube disease among infertility patients.22 The transfer site (uterine versus fallopian tube) per se does not appear to be a predisposing factor in the development of ectopic pregnancies; however, a greater number occur after uterine transfer, because women with bilateral tubal disease are not candidates for GIFT or ZIFT procedures. In approximately 10% of ectopic pregnancy cases, the ectopic embryo develops in conjunction with an ongoing intrauterine pregnancy and requires a termination or surgical removal within the first trimester.22
Preterm delivery, low birth-weight, and small-for-gestational-age infants are more common with ART singleton pregnancies than with natural pregnancies, although the prevalence of admission into the neonatal intensive care unit, duration of infant hospital stay after birth, and the incidence of infant death appear similar (Figure 15-6).23 The difference appears to be a result of infertility per se rather than the ART procedures, because previously infertile women who conceive independent of ART also are at greater risk for preterm delivery.23
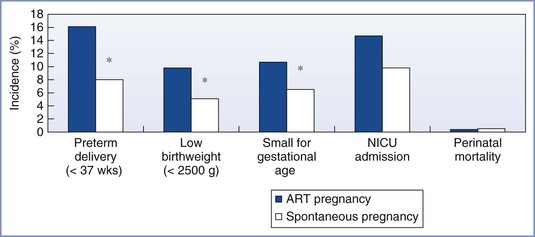
FIGURE 15-6 Outcomes associated with singleton births from assisted reproductive technology (ART) and spontaneous pregnancies in the general population. *P < .05. NICU, Neonatal intensive care unit. (Data from D’Angelo DV, Whitehead N, Helms K, et al. Birth outcomes of intended pregnancies among women who used assisted reproductive technology, ovulation stimulation, or no treatment. Fertil Steril 2011; 96:314-20.)
Effects of Anesthesia on Reproduction
General Considerations
In 1987, Boyers et al.24 reported that oocytes recovered by laparoscopic techniques in patients who had received general anesthesia (i.e., isoflurane or enflurane with a 50% nitrous-oxygen mixture) were less likely to be fertilized if the duration of the procedure was prolonged. Specifically, fertilization rates for the first- and last-recovered oocytes were 69% and 54%, respectively, when the difference in exposure time exceeded 5 minutes. The investigators advanced the following two plausible explanations for this difference: (1) the acidification of follicular fluid by intraperitoneal carbon dioxide and (2) the effects of anesthesia. This study prompted an assessment of anesthetic agents and techniques used during ART procedures.
Ideally, anesthetic techniques and agents used for ART procedures should not interfere with oocyte fertilization or early embryo development and implantation. Although anesthetic agents have been reported to interfere with some aspects of reproductive physiology in some species under certain conditions, the literature must be interpreted with caution. For example, one study concluded that oocyte cleavage rates were significantly lower with general anesthesia than with epidural anesthesia.25 However, a laparoscopic (instead of transvaginal) retrieval method was used in the general anesthesia group, and carbon dioxide pneumoperitoneum may significantly decrease both follicular fluid pH and oocyte fertilization rates. Another report commented on the effects of different anesthetic techniques, but it did not disclose the actual anesthetic agents that were administered in the study.26 In addition, conclusions based on animal data may not reflect the human experience owing to interspecies and assay method differences.27
Assessment of specific anesthetic drugs must also be interpreted in context; relevant factors include (1) the method of administration, (2) dose of anesthetic agents, (3) combination with other drugs, (4) timing of administration, and (5) duration of exposure. For example, local anesthetic agents yield dissimilar pharmacokinetic profiles when administered via paracervical, epidural, and intrathecal techniques. Anesthetic agents may also affect unfertilized oocytes and fertilized embryos differently; thus, studies of anesthetic agents used for a GIFT (prefertilization) procedure should not be directly compared with studies of agents used for a ZIFT (postfertilization) procedure. Finally, significantly greater free concentrations of certain agents (e.g., bupivacaine) exist during ART stimulation because of a decrease in plasma protein binding capacity.28 Thus, when selecting anesthetic techniques or agents for an ART procedure, the clinician should weigh their known benefits (e.g., greater hemodynamic stability, less nausea, less psychomotor impairment) and hypothetical risks (e.g., lower delivery rates).
Local Anesthetic Agents
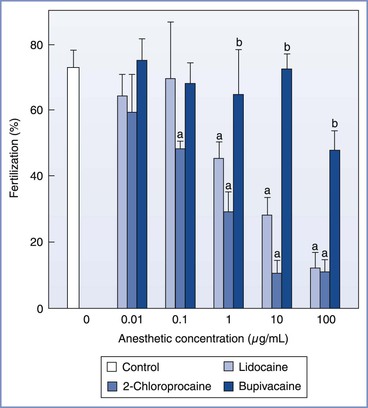
In animal models, the effect of local anesthetic agents on reproductive physiology appears to be related to the agent, timing, and dose of exposure. Using mouse oocytes incubated for 30 minutes in culture media with known concentrations of lidocaine, bupivacaine, or 2-chloroprocaine, Schnell et al.29 demonstrated that lidocaine and 2-chloroprocaine adversely affected both fertilization and embryo development at concentrations of 1.0 and 0.1 µg/mL, respectively (Figures 15-7 and 15-8). In contrast, bupivacaine produced adverse effects only at the greatest concentration studied (100 µg/mL). Similarly, Del Valle et al.30 demonstrated that after 48 hours of culture, 24% of mouse embryos exposed to lidocaine 10 µg/mL, in comparison with none in the control group, showed evidence of degeneration. Finally, Ahuja et al.31 noted that hamster oocytes exposed to procaine or tetracaine demonstrated impaired zona reactions, potentially allowing additional sperm to enter the oocyte and create abnormal chromosomal numbers (polyploidy).