Chapter 37
Fever and Infection
Scott Segal MD, MHCM
Chapter Outline
Fever
Definition and Pathophysiology
In 1868, Carl Wunderlich analyzed more than 1 million axillary temperature measurements from 25,000 patients.1 He concluded that the average normal temperature of healthy adults was 37° C (98.6° F). However, he found a range of temperatures, with a nadir of 36.2° C between 2:00 and 8:00 AM and a zenith of 37.5° C between 4:00 and 9:00 PM. He also observed that women had slightly higher mean temperatures than men. A 1992 study using modern oral thermometers largely confirmed Wunderlich’s original data.2
Well-regulated temperature results from hypothalamic integration of afferent thermal information from the skin, spinal cord, and other sites within the central nervous system (CNS). When this integrated temperature deviates from normal, thermoregulatory responses are triggered.3 In humans, the first (and least metabolically “expensive”) response to temperature perturbations is behavioral (e.g., moving to a different environment, putting on appropriate clothing, adjusting room temperature). Such responses obviously are unavailable to an anesthetized patient, although some may be implemented by those caring for the patient. Further responses to temperature perturbations are mediated by the autonomic nervous system. Hypothermia prompts vasoconstriction in peripheral tissues to decrease skin blood flow, decrease heat loss, and retain heat in the core compartment. If vasoconstriction is not adequate to prevent hypothermia, thermoregulatory shivering is triggered to increase heat production. The CNS controls the metabolic activity of skeletal muscle, which converts chemical energy into heat by shivering.
Increased body temperature initially prompts vasodilation. This vasodilation is passive. It results from the release of sympathetic tone, and it is observed in unanesthetized adults exposed to a hot environment before any significant change in central temperature occurs. If vasodilation is not adequate to prevent hyperthermia, thermoregulatory sweating occurs, which increases evaporative heat loss.
An abnormal body temperature can result from drugs or diseases that either change thermoregulatory thresholds or impair thermoregulatory responses. Hypothalamic activity and fever may be triggered by endogenous pyrogens released from immune effector cells in response to invasion by microorganisms (Figure 37-1). Although no single endogenous pyrogen has been conclusively identified as the mediator of the febrile response, tumor necrosis factor seems capable of reproducing many components of the febrile response.4 Endogenous pyrogen activity appears to depend largely on increased endothelial cell production of prostaglandins. Of interest, many of these substances help mediate uterine activity and parturition.5 The central neural pathways leading to changes in thermoregulatory physiology are increasingly well understood and involve pathways coordinated in the preoptic area and projecting to the dorsomedial hypothalamus or to the rostral medullary raphe region.3
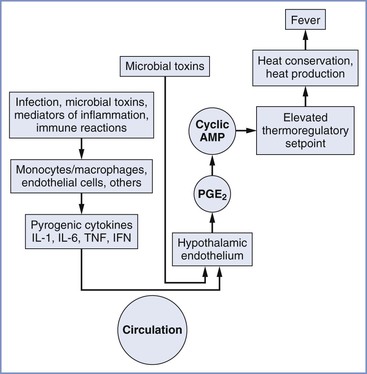
FIGURE 37-1 Chronology of events in the pathophysiology of fever. AMP, adenosine-5′-monophosphate; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; PGE2, prostaglandin E2. (From Longo DL, Fauci AS, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 18th edition. New York, McGraw-Hill, 2012. Available at http://www.accessmedicine.com/. Accessed July 2013.)
Clinically, temperature measurements greater than 38° C represent fever. During episodes of fever, the thermoregulatory set point is elevated and the normal thermoregulatory mechanisms are used to maintain the elevated temperature. However, there are circumstances in which an abnormally high temperature is measured in the absence of a change in thermoregulatory set point, such as when thermoregulatory responses to hyperthermia are prevented (e.g., block of sympathetically mediated sweating) or overwhelmed (e.g., immersion in hot water, malignant hyperthermia).
The fetus, by virtue of its intra-abdominal location, has a unique problem with heat elimination. The only anatomic routes for egress of heat are the fetal skin surface (through the amniotic fluid) or the uteroplacental circulation. Evidence suggests that the fetus relies on heat exchange across the uteroplacental circulation to dissipate most of its metabolic heat. The normal fetus maintains a temperature that is approximately 0.5° C to 0.75° C higher than maternal temperature.6–8
Consequences of Maternal Fever and Infection
Maternal-fetal infection is associated with increased perinatal morbidity.9,10 The increased morbidity is the result of many factors, including preterm delivery (perhaps related to an increased release of prostaglandins) and direct effects of the infection. Fever, irrespective of its etiology, may be harmful to the fetus, although the mechanisms of injury may differ with various causes. Conversely, some infections may be harmful without producing fever. Infection is the most common cause of fever and involves liberation of inflammatory cytokines, which are implicated in the pathogenesis of many fetal and neonatal injuries (see Chapter 10).11,12 Noninfectious inflammatory fever, also leading to elevated cytokines, may complicate neuraxial analgesia in the absence of clinical infection (see later discussion).
Experimental evidence suggests that extreme levels of hyperthermia, independent of infection or inflammation, may have a deleterious effect on the fetus. Morishima et al.13 reported increased uterine activity and fetal deterioration during maternal hyperthermia produced by radiant heat in anesthetized baboons. However, the extreme degree of hyperthermia (approximately 41.7° C) employed in this study produced maternal as well as fetal deaths. Such extreme hyperthermia exceeds the modest fever that often occurs clinically; thus the clinical relevance of this study is unclear. Similarly, Cefalo and Hellegers14 demonstrated fetal deterioration at levels of hyperthermia that produced maternal cardiovascular collapse in anesthetized gravid ewes. However, the investigators also observed increased umbilical blood flow with clinically relevant, mild to moderate hyperthermia (0.5° C to 1.5° C above baseline). They suggested that increased umbilical blood flow in response to moderate degrees of hyperthermia might be beneficial to the fetus by increasing oxygen delivery and heat removal. Harris et al.15 demonstrated the preservation of fetal oxygenation and acid-base status during moderate degrees of fever (approximately 1° C above baseline) produced by the injection of bacterial pyrogen in awake pregnant ewes. However, they also observed an increase in fetal heart rate (FHR) and a greater incidence of fetal arrhythmias during fever.
Nonetheless, in humans, hot tub and sauna use in pregnancy has been linked epidemiologically to neural tube defects in the fetus with attributable risk comparable to that observed with febrile illness.16 Spontaneous abortion17 and major structural birth defects18 have similarly been associated with the frequency of use of hot tubs and saunas. Neonatal hypoxic encephalopathy has been observed after prolonged immersion in 39.7° C water during otherwise uncomplicated labor.19
Epidemiologic evidence suggests that mild maternal intrapartum fever may not be as benign as has been assumed on the basis of animal studies. Macauley et al.8 measured fetal scalp temperature in utero using a modified intrauterine pressure catheter. They concluded that fetal core temperature may exceed 40° C in some febrile women (Figure 37-2). Lieberman et al.20 retrospectively reviewed the records of 1218 nulliparous women with singleton, term pregnancies in spontaneous labor who were afebrile on admission. They found fever (> 38° C) in 10% of the patients, nearly all of whom had received epidural analgesia. One-minute Apgar scores less than 7 and hypotonia were more common in the newborns of febrile mothers. Fever higher than 38.3° C was associated with more frequent requirement for bag-and-mask ventilation in the delivery room and need for supplemental oxygen in the nursery. There was also a nonsignificant increase in the incidence of neonatal seizures.20 The same group performed a case-control study of unexplained neonatal seizures in term infants and found a strong association with intrapartum fever and seizures (odds ratio [OR], 3.4).21 A similar finding was reported by Perlman,22 who found a high incidence of maternal fever among a cohort of infants with a 5-minute Apgar score of 5 or less and those requiring resuscitation with chest compressions in the delivery room. The same group found a higher incidence of prolonged positive-pressure ventilation in the delivery room, tracheal intubation, admission to a neonatal intensive care unit, and 5-minute Apgar score less than 6 in febrile newborns born to women with chorioamnionitis compared with afebrile newborns born to women with chorioamnionitis.23 Similarly, Greenwell et al.24 found an association between low-grade fever (37.5° C) and adverse neonatal outcomes, including hypotonia and low 1-minute Apgar scores. More extreme fever (> 38.3° C) was also associated with low 5-minutes Apgar scores, assisted ventilation, and seizures.24
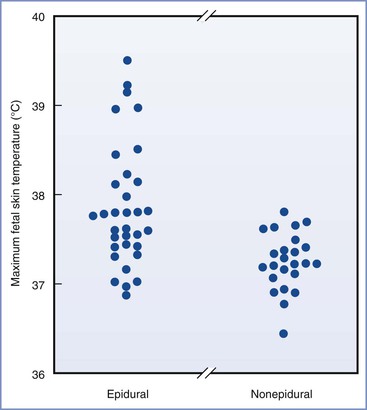
FIGURE 37-2 Maximum fetal scalp temperatures measured in utero in women who selected epidural analgesia and those in women selecting other forms of analgesia. P = .004 epidural compared with nonepidural group. (From Macaulay JH, Bond K, Steer PJ. Epidural analgesia in labor and fetal hyperthermia. Obstet Gynecol 1992; 80:665-9.)
Even more ominous is the suggestion that maternal fever may correlate with neonatal brain injury, particularly when the fever is associated with clinical or pathologically diagnosed chorioamnionitis (see Chapter 10). In several large epidemiologic studies, otherwise unexplained cerebral palsy was two to nine times more common in infants born to mothers with intrapartum fever (> 38° C) than in those born to afebrile mothers.25–29 An equally strong association has been observed between maternal fever and neonatal encephalopathy.30 Even if not apparent at birth or in early infancy, neurologic injury related to maternal fever may appear later in childhood. Dammann et al.31 reported an increased risk for cognitive deficits (> 2 standard deviations below the mean on a nonverbal intelligence scale) at age 9 among children whose mothers were febrile in labor. Fever during pregnancy, not specifically in the intrapartum period, has also been linked to subsequent development of autism32,33 and schizophrenia.34
The mechanism linking neurologic injuries to maternal fever likely involves the liberation of inflammatory cytokines.11,12,29,34 Animal models of chorioamnionitis suggest that fetal brain lesions can be induced by infection and blocked by anti-inflammatory cytokines.35 Infants developing neonatal encephalopathy in the setting of maternal fever do not generally exhibit positive blood cultures, implying that it is neuroinflammation, rather than infection, that causes damage.30,36 It remains unclear whether intrapartum temperature elevation itself can cause neurologic injury, as distinct from underlying infection or other inflammatory processes that cause both elevated inflammatory cytokines and fever.
Fever also produces significant maternal effects. Elevated temperature is associated with increased maternal heart rate, cardiac output, oxygen consumption, and catecholamine production. Evidence has linked fever after cesarean delivery to risk for uterine rupture during subsequent trial of labor after cesarean delivery.37 Women who develop fever are more likely to shiver and experience uncomfortable rigors.38–40 Not surprisingly, obstetricians fearing infection are more likely to treat febrile women with antibiotics.41,42 Even low-grade fever may prompt obstetricians to choose instrumental vaginal or cesarean delivery over expectant labor management. Lieberman et al.43 found a twofold higher incidence of operative vaginal as well as cesarean delivery in a retrospective analysis of nulliparous women who were afebrile at admission but developed fever greater than 37.5° C during labor compared with those who remained afebrile, even after controlling for birth weight, duration of labor, and analgesic choice.
Together, these studies suggest that the fetus acutely tolerates modest degrees of maternal fever. Transient neonatal depression, neonatal seizures, and other neurologic disorders may be associated with inflammatory processes that produce maternal fever, although not clearly with fever per se. Inflammatory processes resulting in fever, and perhaps severe hyperthermia itself, may be risk factors for neonatal brain injury and other abnormalities.
Infections in Pregnant Women
Fever is most often the result of an infectious process. The most common sites for infection in pregnant women are the fetal membranes, urinary tract, respiratory tract, and postpartum uterine cavity.
Chorioamnionitis
Chorioamnionitis is one of the most common infections in pregnant women. It occurs with variable frequency; reported event rates range from 0.5% to 10%, depending on the means of ascertainment and the demographic and obstetric characteristics of the population. In one review, the incidence was 41% for deliveries occurring at less than 27 weeks’ gestation, 15% from 28 to 36 weeks’ gestation, and 2% at term.44 Independent risk factors include low parity, a history of chorioamnionitis in a prior delivery,45 the number of vaginal examinations, duration of total labor, duration of ruptured membranes, and use of internal monitors.45,46 The diagnosis of chorioamnionitis is based on clinical signs, which include temperature greater than 38° C, maternal and/or fetal tachycardia, uterine tenderness, and/or foul-smelling amniotic fluid.9 Unfortunately, the laboratory diagnosis of chorioamnionitis is neither sensitive nor specific and may not correlate with the clinical presentation.9,47–51 Moreover, the classic clinical signs of chorioamnionitis often are absent. Goodman et al.51 reviewed the records of 531 women with pathologically proven chorioamnionitis. They found that only 10% of the patients had abdominal tenderness and only 1% had foul-smelling amniotic fluid. Moreover, histologic evidence of chorioamnionitis can develop without producing clinical signs or symptoms.52,53
In most cases, bacteria gain access to the amniotic cavity and the fetus by ascending through the cervix after rupture of the membranes. Chorioamnionitis develops in a significant number of parturients with premature rupture of the membranes. Alternatively, infectious agents present in the maternal circulation may undergo transplacental transport and gain access to the amniotic cavity.9 Similar to other pelvic infections, chorioamnionitis often is polymicrobial in origin and bacteria normally present in the genital tract most likely are responsible for infections. Bacteroides species, group B streptococci, Mycoplasma and Ureaplasma species, and Escherichia coli are organisms commonly isolated from the amniotic fluid of parturients with chorioamnionitis.9,54 Maternal bacteremia occurs in 7.5% to 12% of women with the clinical diagnosis of chorioamnionitis.9,47–51,55 Candida species occasionally cause chorioamnionitis, especially in preterm labors, and have been associated with severe sequelae, including maternal sepsis and fetal demise.56 Ureaplasma urealyticum has been implicated in intra-amniotic infections even with intact membranes, and infection with this organism is associated with preterm labor.57
Maternal complications of chorioamnionitis include preterm labor,58 placental abruption,59 postpartum infection,60 uterine atony,61 postpartum hemorrhage,62 peripartum hysterectomy,63 sepsis, and death. A large prospective observational study found uterine atony, maternal blood transfusion, septic pelvic thrombophlebitis, pelvic abscess, and maternal admission to the intensive care unit to be independently associated with chorioamnionitis.64 In addition, several studies have noted an increased incidence of cesarean delivery for dystocia in women with chorioamnionitis.* Some investigators have suggested that infection adversely affects uterine contractility and contributes to an increased risk for cesarean delivery.48 However, in some cases, chorioamnionitis may represent an ascending infection developing late in a labor that is already prolonged and dysfunctional.55 Satin et al.55 observed no increase in the incidence of cesarean delivery when chorioamnionitis was diagnosed before the administration of oxytocin. However, they observed a 44% incidence of cesarean delivery when the diagnosis was made after the administration of oxytocin. Thus, the presence of chorioamnionitis may be seen as influencing the clinical decision making of the obstetrician, rather than a direct physiologic cause of dystocia.
Neonatal complications of chorioamnionitis include pneumonia, meningitis, sepsis, and death.9,47 A strong association between chorioamnionitis and cerebral palsy has been identified.25,29,65,66 Meta-analyses of more than 20 studies have demonstrated relative risks of cerebral palsy ranging from 1.9 to 4.7 in preterm and term infants born to mothers with clinical chorioamnionitis.26,66,67 Neonatal stroke has also been linked to chorioamnionitis.68 Chorioamnionitis has been linked epidemiologically to cystic periventricular leukomalacia, which often produces devastating neurologic impairment in the child.66,69 The link between maternal infection and neurologic injury in the neonate appears related to intra-amniotic infection or inflammation, particularly when there is evidence of fetal systemic inflammation (funisitis).29,65
The effect of intra-amniotic inflammation on the fetal lung is complex. Elevated amniotic cytokines may reduce the incidence of acute respiratory distress syndrome in preterm neonates by stimulating surfactant production.70 However, chronic lung disease is increased in infants exposed to chorioamnionitis,71 apparently also related to inflammatory mediators.9,72 Studies in African women suggest chorioamnionitis is associated with an increased risk for peripartum vertical human immunodeficiency virus (HIV) transmission from infected mothers.73
Historically, prompt delivery has been the cornerstone of obstetric management of patients with chorioamnionitis. However, Gibbs et al.47 did not identify a correlation between poor maternal or neonatal outcome and the time interval from diagnosis of chorioamnionitis to delivery. They performed cesarean delivery only for standard obstetric indications and not for the diagnosis of chorioamnionitis alone.47 Similarly, a large prospective observational study found no relationship between the duration of infection and most measures of adverse neonatal outcome among 1965 gestations complicated by chorioamnionitis, although low 5-minute Apgar scores and neonatal mechanical ventilation were correlated with duration of chorioamnionitis.64 No recent studies have reinvestigated this practice as it relates to neonatal neurologic injuries. Monitoring of the FHR pattern is indicated in women with the diagnosis of chorioamnionitis. Many fetuses will exhibit mild tachycardia during maternal fever and infection, but this pattern is not highly predictive of neonatal acidemia and, therefore, by itself, is not an indication for immediate delivery.74
For many years, pediatricians requested that obstetricians delay antibiotic therapy until after delivery. They cited the theoretical concern that intrapartum therapy might “obscure the results of neonatal blood cultures.”75 However, studies suggest that early, antepartum treatment results in decreased maternal and neonatal morbidity compared with delayed, postpartum treatment.76,77 Gibbs et al.76 randomized 45 women with intra-amniotic infection to receive intrapartum or postpartum antibiotic therapy with ampicillin and gentamicin. Intrapartum antibiotic therapy resulted in a decreased incidence of neonatal sepsis and a shorter neonatal hospital stay. Mothers who received intrapartum antibiotics also had a shorter hospitalization, fewer days with fever, and a lower peak postpartum temperature than mothers whose antibiotic therapy was delayed until after delivery. Currently, most obstetricians give antibiotics before delivery in women with chorioamnionitis. The early use of antibiotics also may affect the anesthesiologist’s decision regarding the administration of neuraxial labor analgesia or anesthesia (see later discussion).
Urologic Infections
Urinary tract infections are common during pregnancy, although the incidence of asymptomatic bacteriuria may not be higher than in nonpregnant women. Increased concentrations of progesterone cause the relaxation of ureteral smooth muscle. In addition, the gravid uterus causes partial ureteral obstruction. Both factors cause urinary stasis, which increases the risk for urinary tract infection.9,78,79 Furthermore, these physiologic changes increase the likelihood that asymptomatic bladder infection will ascend into the kidneys and produce pyelonephritis. Approximately 1.3% of pregnant women will develop symptomatic cystitis,80 and up to 25% of women with untreated bacteriuria in pregnancy may develop pyelonephritis.81
Acute pyelonephritis is a serious threat to maternal and fetal well-being and complicates approximately 1.4% of pregnancies. Symptoms of acute pyelonephritis include fever, chills, flank pain, and other symptoms of lower urinary tract infection. Laboratory tests reveal pyuria and leukocytosis with a left shift. Pregnant women with pyelonephritis may appear severely ill. Approximately 14% to 17% of pregnant women with pyelonephritis will develop bacteremia during the course of this infection.82,83 Complications may also include anemia, renal insufficiency, and respiratory insufficiency.83 The most common causative organisms are E. coli, gram-positive organisms, Klebsiella, Enterobacter, and Proteus species.83
Hospitalization is generally required to initiate aggressive parenteral antibiotic treatment of this serious maternal infection, although limited data support outpatient treatment for carefully selected patients in the first and second trimester.84 Nevertheless, treatment failures and septic complications have been reported in patients randomized to outpatient therapy in clinical trials.85
Pyelonephritis is associated with an increased risk for preterm labor and delivery in animal models.86 Thus, obstetricians should observe for evidence of preterm labor, although epidemiologic studies have found the incidence to be less than 5%, approximately the same as in the general pregnant population.83,87
Pyelonephritis may be associated with organ dysfunction. Nearly 20% of affected women have transient renal dysfunction88 and the disease may also be complicated by pulmonary injury. Cunningham et al.89 suggested that “this syndrome was probably caused by permeability pulmonary edema, likely mediated by endotoxin-induced alveolar-capillary membrane injury.”89 Towers et al.90 compared 11 pregnant women who had pyelonephritis and pulmonary injury with 119 women who had pyelonephritis only. They observed that fluid overload and the use of tocolytic therapy were the most significant predictive factors associated with pulmonary injury. The authors suggested that “strict management of fluids should occur so that patients do not have fluid overload.”90 Contemporary authors, however, have questioned fluid restriction in pyelonephritis and instead suggest fluid administration sufficient to generate urine output of 30 to 50 mL/h, while observing respiratory rate, oxygen saturation, and symptoms of dyspnea to identify impending respiratory compromise. Respiratory failure should be investigated with chest radiography and arterial blood gas analysis and managed with appropriate respiratory support.9
Hemodynamic alterations may be present even in infected women who do not demonstrate overt signs of sepsis. Twickler et al.91 used ultrasonographic techniques to evaluate the central hemodynamic measurements in 27 pregnant women with uncomplicated pyelonephritis. They found decreased mean arterial pressure (MAP) and systemic vascular resistance (SVR) and increased heart rate and cardiac output compared with measurements obtained from the same patients after they had recovered from their infection.91
Recent reviews from the Cochrane Database and the United States Preventative Services Task Force strongly support the practice of screening and treating pregnant women for asymptomatic bacteriuria, up to 30% of whom will eventually develop pyelonephritis if left untreated.92,93 Treatment is associated with a reduced incidence of pyelonephritis (OR, 0.23) and low-birth-weight infants (OR, 0.66), although not, as had been previously suggested, in the incidence of preterm delivery.93 Definitive choice of antibiotics could not be determined in a recent systematic review,94 but traditional 7-day regimens are likely more effective than single-dose or other abbreviated antibiotic courses.95 Because pyelonephritis in pregnancy is thought to be preventable, its occurrence may be a marker for poor prenatal care; thus it should alert physicians to other potential pregnancy problems.96
Respiratory Tract Infection
Most respiratory tract infections during pregnancy are upper respiratory tract viral infections that do not pose a serious threat to the mother or fetus. Most lower respiratory tract infections are also viral and self-limiting; pneumonia occurs during pregnancy with an incidence approximating that of the nonpregnant population.97 Pregnancy results in a number of changes that may predispose the pregnant woman to the development of serious respiratory tract infection. Hyperemia and hypersecretion are characteristic of the respiratory tract mucosa during pregnancy, and these changes may intensify the effect of the initial infection.98 In the case of a viral infection, the excess secretions may predispose the patient to bacterial superinfection. Immunologic modulation during pregnancy may also predispose to pulmonary infection.99 Furthermore, the increased oxygen consumption, elevation of the diaphragm, and decreased functional residual capacity characteristic of pregnancy may increase the likelihood that infection will result in maternal hypoxemia.
Benedetti et al.100 emphasized the importance of early diagnosis and treatment as well as the direct measurement of maternal oxygenation in cases of pneumonia during pregnancy. Most community-acquired pneumonias in healthy young women are bacterial in origin. Streptococcus pneumoniae is the most common pathogen.100,101 Mycoplasma pneumoniae and influenza are other common pathogens. Legionella pneumophila, Chlamydia, and varicella are less common pathogens in this population. Varicella pneumonia has been associated with maternal and fetal morbidity and up to 40% maternal mortality. Acyclovir has been used successfully to treat varicella pneumonia during pregnancy.102 Pneumocystis jiroveci (formerly Pneumocystis carinii) pneumonia represents the most common cause of death related to acquired immunodeficiency syndrome (AIDS) in pregnancy; mortality is as high as 50%.103
Because morbidity and mortality from influenza are increased in pregnancy, influenza vaccination is strongly recommended for all pregnant patients, irrespective of gestational age.104 The risk for fetal death is increased by maternal influenza infection and is reduced by vaccination.105 The infant is also passively protected for up to 20 weeks after birth.106 Aggressive treatment with antiviral drugs (oseltamivir, zanamivir) reduces the severity of the ensuing illness; in the 2009-2010 H1N1 influenza pandemic, initiation of therapy in the first 2 days of the infection reduced hospitalization and intensive care unit admission.107,108
Postpartum Infection
The most common source of postpartum infection is the genital tract. The urinary tract and less often the breasts or the lungs may also be infected.9 Postpartum uterine infection typically results in fever, malaise, abdominal pain, and purulent lochia. Bacteremia may occur in as many as 5% to 10% of patients with uterine infection after delivery.49,50,109,110 Although obstetricians typically refer to postpartum uterine infection as endometritis, this infection involves the decidua, myometrium, and parametrial tissues. Bacteria that colonize the cervix and vagina gain access to the amniotic fluid during labor, and they may invade devitalized uterine tissue postpartum.
Patients who undergo cesarean delivery are at increased risk for postpartum endometritis compared with similar patients who deliver vaginally.9 Prolonged rupture of membranes and/or prolonged duration of labor increase the incidence of postpartum uterine infection. Prophylactic administration of antibiotics decreases the incidence of postpartum uterine infection and wound infection after cesarean delivery in all women, whether performed electively or emergently.111 No important differences have been identified among various skin preparation solutions used before cesarean delivery.112 Vaginal preparation before cesarean delivery, however, may reduce endometritis.113 Systematic reviews have found insufficient evidence to recommend prophylactic antibiotics for operative vaginal delivery or for manual removal of the placenta after vaginal delivery; however, consideration may be given to prophylactic antibiotics in the setting of complex perineal repairs.114–116 Endometritis typically responds to appropriate antibiotic therapy, and outcomes are generally excellent. However, serious complications (e.g., peritonitis, abscess, septic thrombophlebitis) may rarely occur.9,49 In future pregnancies, women who have experienced endometritis are at increased risk for uterine rupture, and anesthesiologists and obstetricians should demonstrate extra vigilance for this serious complication.37
Sepsis and Septic Shock
Sepsis is a rare, life-threatening complication of maternal infection that complicates approximately 1 in 8000 deliveries.117 Sepsis is defined by the American College of Chest Physicians and Society of Critical Care Medicine as infection that precipitates the systemic inflammatory response syndrome (SIRS), characterized by two or more of the following criteria: hyperthermia, hypothermia, tachycardia, tachypnea, or leukocytosis (see Chapter 55).118 Pregnancy may complicate the diagnosis, because many healthy pregnant women exhibit some of these signs.119 Noninfectious inflammation may also lead to SIRS; in all cases the syndrome appears to be triggered by mediators released from immune effector cells, including tumor necrosis factor, interleukins, and cyclooxygenase metabolites of arachidonic acid.120
Severe sepsis is defined as sepsis with acute organ dysfunction, hypotension, or sepsis-induced hypoperfusion,118 and septic shock is defined as sepsis-induced hypotension that persists despite adequate fluid resuscitation, along with the presence of hypoperfusion abnormalities or organ dysfunction.118 The incidence of sepsis and sepsis-related maternal mortality appears to be rising; it was the most common cause of death (1.13 per 100,000 maternities) in the most recent (2006-2008) Confidential Enquiries into Maternal Death in the United Kingdom report.121
In pregnant women, sepsis typically is associated with gram-negative bacteremia, although it can occur in association with gram-positive aerobic and anaerobic infections.122 Polymicrobial etiology is common,117,123 particularly when the source of sepsis is a pelvic infection.122 Group A β-hemolytic streptococcal infection appears to predominate in fatal cases or those associated with major morbidity, although Clostridium species have been implicated in deaths of several otherwise healthy women undergoing gynecologic or obstetric procedures.124,125 Untreated pneumonia, chorioamnionitis, pyelonephritis, endometritis, wound infection, incomplete abortion, and self-induced abortion may result in maternal sepsis, severe sepsis, and septic shock; case reports have attributed iatrogenic maternal sepsis to amniocentesis,126–129 medical abortion,125 dental procedures, and assisted reproductive procedures.130 Approximately 45% of cases occur postpartum.131 The clinical course is often fulminant. In a series of nearly 100 cases from The Netherlands, more than half of the patients demonstrated signs of severe sepsis within 48 hours of the first sign of infection and 39% did so within 24 hours.131
Studies have suggested that pregnancy makes laboratory animals more susceptible to infection by a number of mechanisms.132 For example, Beller et al.133 infused E. coli B6 lipopolysaccharide (endotoxin) into both nonpregnant and pregnant minipigs. The average duration of survival for the nonpregnant animals was 16 hours, whereas the average duration of survival for the pregnant animals was only 3.5 hours. The pregnant animals suffered more pronounced cardiovascular abnormalities and metabolic acidosis than the nonpregnant animals.
It is unclear whether pregnant women are more susceptible to sepsis than age-matched nonpregnant women. Case series of maternal sepsis have reported overall case-fatality rates of 6% and 7.7%.122,131 This survival rate may reflect the relative youth of pregnant women and the effectiveness of surgical source control for most pelvic infections. Nonetheless, clinical studies have noted that once septic shock develops in pregnant patients, maternal mortality is as high as 30%.134–136
The mainstay of therapy is elimination and/or aggressive treatment of the source of infection with antibiotics and, if indicated, surgical extirpation. Initial antibiotic therapy should include broad-spectrum coverage for bacteria such as E. coli, enterococcus, and anaerobic organisms. A combination of ampicillin, gentamicin, and clindamycin represents an effective regimen, as does a combination of imipenem, cilastatin, and vancomycin.137 A 2012 review of cases from the United Kingdom Center for Maternal and Child Enquiries (CMACE) project suggested initial broad-spectrum antibiotic coverage (e.g., gentamicin with either piperacillin-tazobactam or ciprofloxacin), consultation with an infectious disease specialist as soon as possible, and appropriate adjustment of antibiotic therapy once the causative organism and antibiotic susceptibilities have been identified.138 Local patterns of microbial resistance should be considered when selecting broad-spectrum empirical therapy. In all cases, aggressive antibiotic initiation without delay (within 1 hour of diagnosis) appears important for a favorable outcome.121,139
Only a few reports have described the management of these seriously ill patients. Timezguid et al.122 summarized 66 intensive care unit admissions for sepsis between 1977 and 2008. Seventeen of 22 women with a pelvic source of infection required surgical source control, including termination of pregnancy (n = 5), uterine exploration for removal of placental fragments (n = 6), drainage of a pelvic abscess (n = 5), or hysterectomy (n = 3). Premature termination of pregnancy was more frequent in 12 cases of chorioamnionitis (83%) than in the 32 cases of extrapelvic infections (31%). Vasopressors, mechanical ventilation, and renal replacement therapy were used in 16 (25%), 19 (28%), and 4 (6%) cases, respectively.
Lee et al.135 reviewed 10 cases of septic shock in obstetric patients between 1984 and 1986, which were caused by pelvic infections in 9 cases and mastitis in 1. Eight of the 10 women required inotropic or vasopressor infusions, or both, guided by pulmonary arterial catheterization. Two women died. The primary hemodynamic abnormalities were decreased systemic vascular resistance and depressed myocardial function.
Mabie et al.136 reported 18 cases of pregnancy-associated septic shock that occurred during 11 years in a single institution, an incidence of 1 per 8338 deliveries. The most common causes were pyelonephritis (n = 6), chorioamnionitis (n = 3), endometritis (n = 2), and toxic shock syndrome (n = 2). Five patients (28%) died. The hemodynamic profiles of the patients in this series were similar to those described by Lee et al.135: four of the five patients who died had mildly or severely depressed myocardial function.136 Eight patients underwent at least one surgical procedure to control the source of infection.
In most cases, concomitant supportive therapy is required to decrease maternal and fetal morbidity. Physicians must give attention to the maintenance of maternal oxygenation, circulation, and coagulation. Most authorities suggest following the Surviving Sepsis guidelines for treatment of sepsis.121,138,139 The guidelines recommend goal-directed therapy aiming to achieve a central venous pressure (CVP) between 8 and 12 mm Hg and a MAP of 65 mm Hg, urine output of 0.5 mL/kg/h, and mixed venous oxygen saturation 65% or higher (see Chapter 55).139 Pregnant women were excluded from trials that evaluated this goal-directed therapy,140,141 and unfortunately no evidence is available to confirm that this approach offers survival benefit for pregnant or recently delivered women. In nonpregnant patients with septic shock, norepinephrine is considered the first choice vasopressor and may be supplemented with epinephrine or vasopressin to maintain adequate blood pressure.139
Evidence from nonobstetric patients receiving intensive care indicates that aggressive glycemic control may not confer a survival advantage in all critically ill patients, although some evidence suggests that avoiding hyperglycemia improves outcomes in surgical patients.142 Overly tight glycemic targets increase the risk for hypoglycemia; current guidelines recommend treatment to avoid hyperglycemia (> 180 mg/dL), hypoglycemia, and wide swings in glucose levels.139 More intensive glucose control may be indicated in the intrapartum period to achieve optimal neonatal outcomes. Corticosteroid supplementation is indicated only when fluid resuscitation and vasopressor therapy fail to restore hemodynamic stability.139,143
Although the effects of maternal therapy on the fetus should be considered, treatment of the mother has first priority. Often what is best for the mother will be best for the fetus. However, in some cases, maternal sepsis may require preterm delivery, or even delivery before the age of viability, particularly if chorioamnionitis is present. Although the American College of Obstetricians and Gynecologists (ACOG) has stated that “delivery usually is not indicated in the septic pregnant patient in whom the pregnancy is not the source of infection,”137 a multidisciplinary team should consider the mother’s response to initial therapeutic interventions, her vasopressor, oxygen and ventilatory requirements, and the gestational age and fetal well-being when considering the optimal timing and route of delivery.
Epidural Analgesia and Maternal Fever
Incidence
Epidural anesthesia administered for surgery—including cesarean delivery—typically results in hypothermia. This effect occurs because vasodilation produced by the sympathetic neuroblockade causes a redistribution of body heat from the core to the periphery, where it is lost to the environment.144
In 1989, Fusi et al.145 first observed that epidural labor analgesia was associated with progressive intrapartum maternal pyrexia. They reported that the vaginal temperatures of 18 parturients who received epidural analgesia increased approximately 1° C over 7 hours, whereas the temperatures of 15 women who received intramuscular meperidine and metoclopramide remained constant. There was no evidence of infection in any of the women. The authors suggested that epidural analgesia may cause an “imbalance between the heat-producing and heat-dissipating mechanisms.”
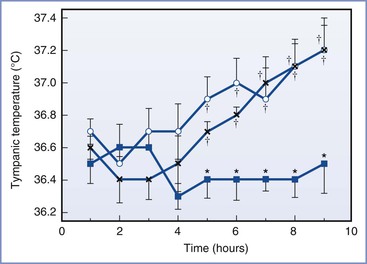
Fusi et al.145 measured vaginal temperature; this measurement may be affected by the sympathectomy and vaginal mucosal vasodilation associated with epidural analgesia. Tympanic membrane temperature should not be affected by the local vasodilation associated with epidural analgesia, and it may provide a more accurate assessment of core temperature. Camann et al.146 studied the effect of epidural analgesia on maternal oral and tympanic membrane temperature measurements in 53 laboring women. They studied three groups of patients; one group received intravenous nalbuphine, one group received epidural bupivacaine only, and one group received epidural bupivacaine with fentanyl. The patients were not randomized to receive either nalbuphine or epidural analgesia; however, the women who requested epidural analgesia were randomized to receive epidural bupivacaine only or epidural bupivacaine with fentanyl. The authors maintained ambient room temperature between 20° C to 22° C. Epidural analgesia did not affect maternal temperature during the first 4 hours of the study. At 5 hours and thereafter, the mean tympanic membrane temperatures were significantly higher in both of the epidural groups than the intravenous nalbuphine group (Figure 37-3). Among women in the bupivacaine-only group, mean tympanic temperature increased from approximately 36.6° C at 1 hour to 37.1° C at 9 hours, a rate of rise of less than 0.07° C per hour. There was no difference between the epidural bupivacaine-only and the epidural bupivacaine-fentanyl groups in maternal tympanic membrane temperature measurements.