Background
In Ohio, the infant mortality rate is above the national average and the black infant mortality rate is more than twice the white infant mortality rate. Having a short interpregnancy interval has been shown to correlate with preterm birth and low birthweight, but the effect of short interpregnancy interval on infant mortality is less well established.
Objective
We sought to quantify the population impact of interpregnancy interval on the risk of infant mortality.
Study Design
This was a statewide population-based retrospective cohort study of all births (n = 1,131,070) and infant mortalities (n = 8152) using linked Ohio birth and infant death records from January 2007 through September 2014. For this study we analyzed 5 interpregnancy interval categories: 0-<6, 6-<12, 12-<24, 24-<60, and ≥60 months. The primary outcome for this study was infant mortality. During the study period, 3701 infant mortalities were linked to a live birth certificate with an interpregnancy interval available. We calculated the frequency and relative risk of infant mortality for each interval compared to a referent interval of 12-<24 months. Stratified analyses by maternal race were also performed. Adjusted risks were estimated after accounting for statistically significant and biologically plausible confounding variables. Adjusted relative risk was utilized to calculate the attributable risk percent of short interpregnancy intervals on infant mortality.
Results
Short interpregnancy intervals were common in Ohio during the study period. Of all multiparous births, 20.5% followed an interval of <12 months. The overall infant mortality rate during this time was 7.2 per 1000 live births (6.0 for white mothers and 13.1 for black mothers). Infant mortalities occurred more frequently for births following short intervals of 0-<6 months (9.2 per 1000) and 6-<12 months (7.1 per 1000) compared to 12-<24 months (5.6 per 1000) ( P < .001 and <.001). The highest risk for infant mortality followed interpregnancy intervals of 0-<6 months (adjusted relative risk, 1.32; 95% confidence interval, 1.17–1.49) followed by interpregnancy intervals of 6-<12 months (adjusted relative risk, 1.16; 95% confidence interval, 1.04–1.30). Analysis stratified by maternal race revealed similar findings. Attributable risk calculation showed that 24.2% of infant mortalities following intervals of 0-<6 months and 14.1% with intervals of 6-<12 months are attributable to the short interpregnancy interval. By avoiding short interpregnancy intervals of ≤12 months we estimate that in the state of Ohio 31 infant mortalities (20 white and 8 black) per year could have been prevented and the infant mortality rate could have been reduced from 7.2-7.0 during this time frame.
Conclusion
An interpregnancy interval of 12-60 months (1-5 years) between birth and conception of next pregnancy is associated with lowest risk of infant mortality. Public health initiatives and provider counseling to optimize birth spacing has the potential to significantly reduce infant mortality for both white and black mothers.
Introduction
Infant mortality is defined as a death of a baby in the first year of life and the infant mortality rate (IMR) is the number of infant deaths for every 1000 live births. Infant mortality is often used as an indicator of the overall health of a community or population because it is a reflection of a combination factors including maternal health, quality and access to medical care, and socioeconomic status. The US IMR has improved in recent years but remains one of the highest among developed countries. Unfortunately in Ohio the IMR is higher than the national average. In 2014 there were 955 infant deaths with an IMR of 6.8, which is 13% higher than the national average of 6.0. In the same year in Cincinnati the IMR was even higher at 8.8 with a 10-year average IMR of 10.3 (Ohio Department of Health, 2014). Even more striking is the disparity in the IMR by race with the black IMR more than twice the white IMR (14.3 vs 5.3 in 2014).
The leading causes of infant mortality are well documented and have been shown to be related to preterm birth, small-for-gestational-age (SGA) births, sleep-related deaths, and birth defects. There are a multitude of risk factors that contribute to these leading causes of infant mortality; some, such as smoking, may contribute to >1 of the leading causes. Smoking throughout pregnancy results in an increased risk of preterm birth and fetal growth restriction. Additionally, Dietz et al estimate that 5.3-7.7% of preterm deliveries, 23.2-33.6% of sudden infant death syndrome, and 5.0-7.3% of preterm-related deaths are directly attributable to prenatal smoking. Other major risk factors that contribute to infant mortality include maternal health status and social determinants of health including racism, poverty, poor nutrition, and poor education. An additional risk factor that has consistently been correlated with many of the adverse perinatal outcomes associated with infant mortality is having a short interpregnancy interval (IPI). Specifically, a short IPI carries an increased risk of preterm birth, SGA births, and birth defects. Similarly to smoking, having a short IPI likely has a complex multifactorial effect on infant mortality.
The exact mechanism by which having a short IPI leads to adverse perinatal outcomes is not entirely understood, but it has been shown that short IPI increases the risk of uterine rupture in women attempting vaginal birth after cesarean, premature rupture of membranes, endometritis, third-trimester bleeding, placenta previa, placental abruption, maternal death, and anemia. One popular explanation in the literature that may lead to these adverse outcomes is the possibility of maternal nutritional depletion syndrome. This hypothesis proposes that closely spaced pregnancies worsen the maternal nutritional status because of the limited recovery time after the stress of the first pregnancy and subsequent postpartum breast-feeding. This impaired maternal nutritional status then increases the risk of adverse perinatal outcomes.
A short IPI may subsequently lead to an increased risk of infant mortality via the adverse outcomes that place infants at an increased risk of death or via another unidentified mechanism. Regardless, reduction and prevention of short IPIs is a potentially achievable intervention to reduce the IMR especially in high-risk groups. Many of the studies that looked at the effect of IPI on infant mortality thus far looked at different IPIs, failed to control for appropriate confounding variables, had small sample sizes, are now out of date, or were either done outside of the United States or in United States with a notably different patient population from that seen in the Midwest. This study examines the effects of IPI on infant mortality using linked recent Ohio Birth and Infant Death Certificate records.
Materials and Methods
We performed a statewide population-based retrospective cohort study of all births (n = 1,131,070) and infant deaths (n = 8152) using linked Ohio birth and infant death records from January 2007 through September 2014. The protocol for this study was approved by the human subjects institutional review board of the Ohio Department of Health and a deidentified data set extracted from both live birth and infant death certificates was provided for this analysis. This study was exempt from review by the institutional review board at the University of Cincinnati in Ohio.
IPI was defined as the time from the most recent prior birth to conception of the index birth. To calculate the IPI, we first determined the birth-to-birth interval in weeks. Then we subtracted the gestational age of the current index birth (in weeks) from the birth-to-birth interval. For this study we stratified IPI into 5 categories: 0-<6, 6-<12, 12-<24, 24-<60, and ≥60 months. The 12-<24 month category was chosen as the referent group based on data showing that an IPI of 12-<24 months is associated with the lowest risk of adverse outcomes including SGA newborns and preterm birth –known risk factors for infant mortality. The primary outcome for this study was infant mortality. Infant mortality was additionally stratified by maternal race, which is documented on the live birth record.
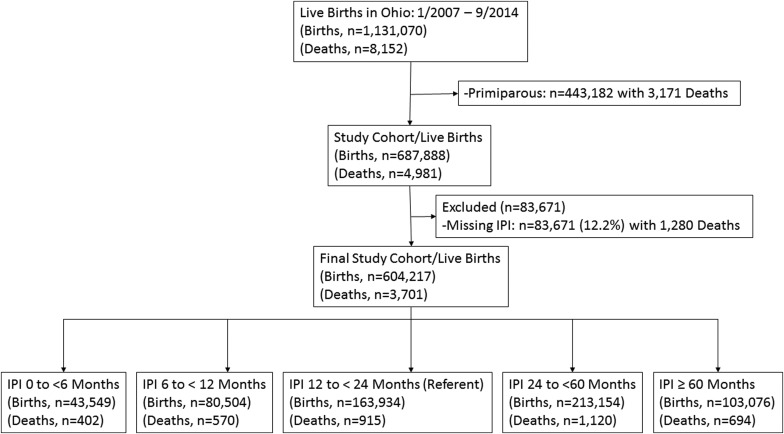
During the study period, there were 1,131,070 live births and 8152 infant deaths ( Figure 1 ). These births were composed of 443,182 primiparous women with 3171 infant deaths and 687,888 multiparous women with 4981 infant deaths. All births were used to compare baseline sociodemographic, maternal pregnancy-related characteristics, and birth-related outcomes between IPI categories and to primiparous women. Additionally, all births were utilized to compare those pregnancies that did or did not subsequently result in an infant death. When looking specifically at the impact of IPI on infant mortality, we excluded births to primiparous women (n = 443,182 with 3171 infant deaths) and births missing the required data to calculate an IPI (n = 83,671 with 1280 infant deaths). After these exclusions there were 604,217 live births to multiparous women with 3701 linked infant deaths available to examine the effect of IPI on infant mortality. The women with missing data required to calculate an IPI comprised 12.2% of the births in multiparous women. We calculated the frequency and relative risk (RR) of infant mortality for each IPI compared to a referent IPI category of 12-<24 months. Stratified analyses by maternal race were also performed. Adjusted RR were estimated after accounting for statistically significant and biologically plausible confounding variables. For overall infant mortality the final model included adjustment for marital status, Medicaid use, tobacco use, maternal age, and race. For white and black infant mortality the model included adjustment for marital status, Medicaid use, tobacco use, and maternal age. Adjusted RR was utilized to calculate the attributable risk percent of IPI on infant mortality among births that occurred following each of the IPIs.
Statistical analysis was performed using χ 2 test for comparisons of dichotomous variables and analysis of variance for continuous variables. Multivariate logistic regression analysis with backward selection of covariates was used to estimate adjusted RR. Significant differences were defined as comparisons with P value <.05 and 95% confidence interval (CI) not inclusive of the null value 1.0. Statistical analyses were performed using software (STATA, Release 12; StataCorp, College Station, TX).
Materials and Methods
We performed a statewide population-based retrospective cohort study of all births (n = 1,131,070) and infant deaths (n = 8152) using linked Ohio birth and infant death records from January 2007 through September 2014. The protocol for this study was approved by the human subjects institutional review board of the Ohio Department of Health and a deidentified data set extracted from both live birth and infant death certificates was provided for this analysis. This study was exempt from review by the institutional review board at the University of Cincinnati in Ohio.
IPI was defined as the time from the most recent prior birth to conception of the index birth. To calculate the IPI, we first determined the birth-to-birth interval in weeks. Then we subtracted the gestational age of the current index birth (in weeks) from the birth-to-birth interval. For this study we stratified IPI into 5 categories: 0-<6, 6-<12, 12-<24, 24-<60, and ≥60 months. The 12-<24 month category was chosen as the referent group based on data showing that an IPI of 12-<24 months is associated with the lowest risk of adverse outcomes including SGA newborns and preterm birth –known risk factors for infant mortality. The primary outcome for this study was infant mortality. Infant mortality was additionally stratified by maternal race, which is documented on the live birth record.
During the study period, there were 1,131,070 live births and 8152 infant deaths ( Figure 1 ). These births were composed of 443,182 primiparous women with 3171 infant deaths and 687,888 multiparous women with 4981 infant deaths. All births were used to compare baseline sociodemographic, maternal pregnancy-related characteristics, and birth-related outcomes between IPI categories and to primiparous women. Additionally, all births were utilized to compare those pregnancies that did or did not subsequently result in an infant death. When looking specifically at the impact of IPI on infant mortality, we excluded births to primiparous women (n = 443,182 with 3171 infant deaths) and births missing the required data to calculate an IPI (n = 83,671 with 1280 infant deaths). After these exclusions there were 604,217 live births to multiparous women with 3701 linked infant deaths available to examine the effect of IPI on infant mortality. The women with missing data required to calculate an IPI comprised 12.2% of the births in multiparous women. We calculated the frequency and relative risk (RR) of infant mortality for each IPI compared to a referent IPI category of 12-<24 months. Stratified analyses by maternal race were also performed. Adjusted RR were estimated after accounting for statistically significant and biologically plausible confounding variables. For overall infant mortality the final model included adjustment for marital status, Medicaid use, tobacco use, maternal age, and race. For white and black infant mortality the model included adjustment for marital status, Medicaid use, tobacco use, and maternal age. Adjusted RR was utilized to calculate the attributable risk percent of IPI on infant mortality among births that occurred following each of the IPIs.
Statistical analysis was performed using χ 2 test for comparisons of dichotomous variables and analysis of variance for continuous variables. Multivariate logistic regression analysis with backward selection of covariates was used to estimate adjusted RR. Significant differences were defined as comparisons with P value <.05 and 95% confidence interval (CI) not inclusive of the null value 1.0. Statistical analyses were performed using software (STATA, Release 12; StataCorp, College Station, TX).
Results
Short IPIs were common in Ohio during the study period with 20.5% of all multiparous births, 20.0% of births to multiparous white mothers, and 23.6% of births to multiparous black mothers following an IPI of <12 months ( Figure 2 ). Although the percentage of births with a short IPI was high in our study, the percentage of births with a short IPI among those that resulted in an infant death was even higher at 34.3%.
When comparing the sociodemographic characteristics of the women with very short (0-<6 months) and short (6-<12 months) IPIs to the referent group (12-<24 months), women with very short and short IPIs are less likely to be married and be a non-Hispanic white female, but more likely to be younger; have less than a high school diploma; have Medicaid; be enrolled in Women, Infants, and Children (WIC); use tobacco; and have <6 prenatal visits ( Table 1 ).
| 0-<6 mo n = 43,549 | 6-<12 mo n = 80,504 | 12-<24 mo n = 163,934 | 24-<60 mo n = 213,154 | ≥60 mo n = 103,076 | Primips | P value | |
|---|---|---|---|---|---|---|---|
| Sociodemographic characteristics | |||||||
| Age, y, mean (SD) | 25.6 (5.2) | 27.2 (5.4) | 28.3 (5.3) | 28.9 (5.2) | 31.8 (4.9) | 25.0 (5.7) | <.001 |
| Married | 47.7% | 63.3% | 70.2% | 64.2% | 53.5% | 49.6% | <.001 |
| ≤High school education | 27.9% | 21.1% | 15.7% | 13.7% | 12.4% | 15.7% | <.001 |
| Medicaid | 56.6% | 41.7% | 34.1% | 38.3% | 43.1% | 36.7% | <.001 |
| WIC | 58.0% | 42.3% | 34.8% | 38.8% | 45.4% | 43.3% | <.001 |
| Tobacco use | 29.6% | 22.1% | 19.2% | 23.9% | 31.1% | 22.9% | <.001 |
| ≤5 Prenatal visits | 19.0% | 13.5% | 9.8% | 8.0% | 7.6% | 7.3% | <.001 |
| Non-Hispanic white | 69.4% | 76.6% | 80.5% | 76.5% | 70.6% | 77.5% | <.001 |
| Non-Hispanic black | 23.1% | 17.0% | 13.3% | 15.9% | 20.7% | 15.2% | <.001 |
| Hispanic | 5.7% | 4.4% | 3.9% | 4.8% | 5.9% | 4.0% | <.001 |
| Other | 1.5% | 1.8% | 2.1% | 2.6% | 2.6% | 3.1% | <.001 |
| Maternal pregnancy characteristics | |||||||
| Parity, median (IQR) | 2 (0–4) | 1 (0–1) | 1 (0–1) | 1 (0–1) | 1 (0–1) | 0 (0–0) | <.001 |
| Prior preterm birth | 8.4% | 6.7% | 6.0% | 6.3% | 6.8% | 0.0% | <.001 |
| Underweight, BMI <18.5 | 3.7% | 4.2% | 4.3% | 3.9% | 3.1% | 5.1% | <.001 |
| Obese, BMI >30 | 29.6% | 24.8% | 22.8% | 26.1% | 31.4% | 20.4% | <.001 |
| Prepregnancy BMI (SD) | 27.4 (6.9) | 26.6 (6.7) | 26.2 (6.6) | 26.8 (6.9) | 27.8 (7.3) | 25.8 (6.5) | <.001 |
| Pregestational diabetes | 0.7% | 0.7% | 0.7% | 0.9% | 1.5% | 0.8% | <.001 |
| Chronic hypertension | 1.8% | 1.6% | 1.6% | 2.1% | 3.9% | 1.8% | <.001 |
| Gestational weight gain, lb (SD) | 27.6 (17.2) | 28.9 (16.8) | 30.1 (16.6) | 30.2 (17.7) | 30.4 (19.0) | 34.4 (17.6) | <.001 |
| Gestational diabetes | 5.2% | 5.0% | 5.1% | 6.5% | 9.7% | 5.1% | <.001 |
| Preeclampsia | 3.7% | 3.6% | 3.6% | 4.4% | 6.3% | 5.8% | <.001 |
| Birth characteristics | |||||||
| Cesarean delivery | 27.1% | 27.8% | 28.9% | 31.6% | 34.2% | 30.2% | <.001 |
| Male infant | 51.0% | 51.1% | 51.0% | 51.2% | 51.0% | 51.3% | .635 |
| Gestational age, wk, mean (SD) | 38.4 (2.84) | 38.6 (2.58) | 38.6 (2.35) | 38.5 (2.38) | 38.3 (2.69) | 38.7 (2.75) | <.001 |
| Birthweight, g, mean (SD) | 3210 (606) | 3319 (574) | 3357 (560) | 3320 (574) | 3239 (625) | 3226 (605) | <.001 |
| SGA births <10% | 11.3% | 8.7% | 7.8% | 8.8% | 11.0% | 12.4% | <.001 |
| LGA births >90% | 8.9% | 11.4% | 12.4% | 11.6% | 10.6% | 7.8% | <.001 |
| Preterm birth | 14.7% | 11.6% | 10.1% | 11.0% | 14.0% | 11.8% | <.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


