Chapter 30
The Difficult Airway
Risk, Assessment, Prophylaxis, and Management
Robin Russell MBBS, MD, FRCA, Mansukh Popat MBBS, FRCA
Chapter Outline
Risk
Definitions
A difficult airway can be defined in several ways. A practitioner may be said to encounter a difficult airway when he or she experiences difficulty providing adequate maintenance or protection of the airway that leads to hypoxemia or soiling of the tracheobronchial tree.1 This definition includes difficulty in providing ventilation via a facemask or supraglottic airway (e.g., laryngeal mask airway [LMA]) or tracheal intubation. The American Society of Anesthesiologists (ASA) Task Force on Management of a Difficult Airway defines a difficult airway as the clinical situation in which a conventionally trained anesthesiologist experiences difficulty with facemask ventilation of the upper airway, difficulty with tracheal intubation, or both.2
The prevalence of difficult facemask ventilation is dependent on the definition. In one study,3 5% of 1502 nonpregnant patients experienced difficulty in facemask ventilation, which was defined as an oxyhemoglobin saturation value less than 92%.3 A multivariate analysis identified five independent risk factors for difficult facemask ventilation: (1) age older than 55 years, (2) body mass index (BMI) greater than 26 kg/m2, (3) presence of a beard, (4) lack of teeth, and (5) a history of snoring. Impossible mask ventilation, defined as an inability to exchange air during bag-mask ventilation despite multiple providers, airway adjuncts, and neuromuscular blockade, was reported in 77 of 50,000 (0.15%) nonobstetric anesthetic procedures.4 Independent predictors of impossible mask ventilation were previous neck irradiation, male gender, diagnosis of sleep apnea, and a Mallampati class III or IV (see later discussion).4 Difficult laryngeal mask ventilation may be defined as the inability within three attempts of device insertion to produce expired tidal volumes more than 7 mL/kg (leak pressure > 15 to 20 cm H2O).1 In a study of 11,910 nonobstetric patients,5 the incidence of difficult laryngeal mask ventilation was 0.19%.
Although failed tracheal intubation is a tangible endpoint, defining difficult intubation is more complex. Difficulty may be encountered because of failure to visualize the glottis (difficult laryngoscopy) or due to an anatomic laryngeal or tracheal abnormality. Difficulty has been variously defined by (1) the time taken to intubate, (2) the number of attempts, (3) the view at laryngoscopy, and (4) the requirement for special equipment.
Although a dramatic decrease in the number of anesthesia-related deaths has been reported in the Confidential Enquiries into Maternal Deaths in the United Kingdom over the past 40 years, complications from general anesthesia, primarily complications of airway management, continue to be the leading cause of anesthesia-related maternal mortality.6 Similarly, data from the United States have demonstrated a higher case-fatality rate with general anesthesia compared with neuraxial anesthesia.7 Although the development of national guidelines2,8,9 has resulted in a more systematic approach to the management of the difficult airway, deaths directly resulting from anesthesia still occur owing to failures in ventilation, tracheal intubation, or airway management following extubation. Despite widespread use of neuraxial anesthesia for operative delivery, general anesthesia may still be required in emergency situations, if neuraxial anesthesia is contraindicated or patients refuse it, or if neuraxial anesthesia has failed to provide adequate anesthesia.
Incidence and Epidemiology
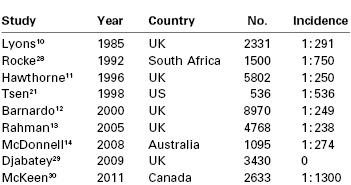
The incidence of failed intubation in obstetrics has long been considered to be approximately 1 in 250 to 300,10–14 which is approximately eight times greater than that in the general population (Table 30-1).15 A number of reasons have been proposed to explain the increased difficulty with obstetric airway management. There are significant physiologic and anatomic changes of pregnancy (see later discussion) affecting the airway, oxygenation, and metabolism. The majority of obstetric general anesthetics are administered for emergency deliveries, often during off hours16; these anesthetic procedures may be conducted by inexperienced anesthesia providers with less proficiency in difficult airway management. Indeed, in one U.K. study,12 the relative risk of failed intubation at emergency compared with elective cesarean delivery was 1.79 (95% confidence interval [CI], 0.61 to 5.26). Excessive cricoid pressure applied by a poorly trained assistant can worsen the glottic view at laryngoscopy,17 as can positioning the parturient with left lateral tilt. Marfin et al.18 proposed that the introduction of disposable airway equipment may adversely affect airway management; single-use, disposable gum elastic bougies are less reliable than reusable devices.
An increase in the incidence of airway-related complications in obstetric patients has been predicted.19,20 The change in maternal demographics, most notably an increase in the prevalence of maternal obesity, may increase the risk for complications from general anesthesia, especially when performed for emergency procedures. With a decrease in the number of cesarean deliveries performed under general anesthesia, trainees have fewer opportunities to become familiar with challenges of the obstetric difficult airway.21–23 Changes in anesthesia training, notably the reduction in trainee working hours and the advent of supraglottic airway (SGA) devices mean that, overall, laryngoscopy and intubation are now less commonly performed than previously. Therefore, the skills required to manage a challenging tracheal intubation are less likely to have been gained before working on the labor and delivery unit without direct supervision. In an editorial, Russell19 suggested that failure to intubate during emergency cesarean delivery may be a self-fulfilling prophecy as practitioners draw on less experience with intubating the trachea during general anesthesia for an emergency cesarean delivery.
The increasing prevalence of maternal obesity is of significant concern. Obese women are at increased risk for obstetric interventions requiring anesthesia24 and are more likely to have unsuccessful neuraxial anesthesia, necessitating the use of general anesthesia for emergency delivery (see Chapter 50). Difficulty with intubation has been reported to occur in 15.5% of the nonobstetric obese population.25 A large Danish cohort study of more than 90,000 nonobstetric patients found that BMI of greater than 35 kg/m2 was a significant risk factor for difficult intubation (odds ratio, 1.34)26; BMI was a more accurate predictor of difficult intubation than weight alone. Data collected from one U.K. region from 1993 to 1998 identified 26 parturients with failed intubation at cesarean delivery; the mean BMI was 33.1 kg/m2.12 Poor head and neck positioning at induction of anesthesia, inappropriate cricoid pressure, and operator anxiety may be responsible for a higher incidence of difficult airway management in obese patients.27
In contrast to some experts, others have questioned whether the rate of difficult and failed intubation is increasing in obstetric anesthesia practice.28–30 A more liberal attitude toward the use of general anesthesia has been suggested to lead to greater familiarity with maternal airway management and subsequent reduced rates of difficulty.29 The ethics of using a technique potentially associated with greater risk for individual harm to reduce the overall incidence of anesthesia-related mortality associated with the technique presents an interesting dilemma. Certainly the presence of experienced anesthesia staff during induction of general anesthesia is recommended and should reduce the morbidity and mortality, and perhaps the frequency, of difficulty with airway management.16 It is hoped that the introduction and widespread acceptance of simulation training in obstetrics31 will lead to improvement in staff performance during critical events such as difficult airway management.
Maternal Morbidity and Mortality
For many years the U.K. Confidential Enquiries into Maternal Deaths have reported thromboembolism, hypertensive disease, and hemorrhage as the leading causes of maternal mortality. In the most recent report, covering the 2006 to 2008 triennium, pregnancy-related mortality from anesthetic causes was the 11th most common cause, accounting for 2.7% of maternal deaths.6 In the United States between 1991 and 2002, 1.6% of maternal deaths were related to complications of anesthesia care, representing a 59% reduction in anesthesia-related mortality compared with data from 1979 to 1990.7,32 Experience from both countries demonstrates dramatic improvements in anesthesia-related maternal mortality in the past three decades. This improvement likely reflects the tremendous efforts that been made by national anesthesia organizations in defining standards of care that lead to improved maternal safety.
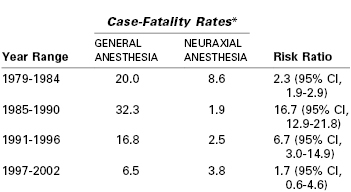
Compared with neuraxial anesthesia, general anesthesia is associated with a greater risk for maternal mortality (Table 30-2, see Chapter 40).7 Using data from the Centers for Disease Control and Prevention (CDC), the estimated case-fatality risk ratio for general compared with neuraxial anesthesia was 16.7 between the years 1985 and 1990.32 However, the estimated risk ratio for the period between 1997 and 2002 was only 1.7 (95% CI, 0.6 to 4.6, P = .2).32 Improvements in monitoring and the publication of algorithms for difficult airway management have been suggested to account for the reduction in mortality from general anesthesia. The case-fatality risk for general anesthesia from the earlier period may have overstated the relative risk because the accuracy of data was questionable and it is likely that general anesthesia was used for more complex cases for which mortality was expected to be greater.33
Unfortunately, maternal deaths directly attributable to general anesthesia are still reported.6 Although protocols for the management of a difficult airway are now ubiquitous, they are not always followed.12,34
Hypoventilation and airway obstruction after extubation are now increasingly recognized as causes of maternal mortality.6,35 In Michigan between 1985 and 2003, eight maternal deaths were believed related to anesthesia care; all deaths occurred during emergence from general anesthesia or the recovery period, and six of the eight patients were obese. System errors in which the care of the patient did not meet recognized standards were identified in five of the eight cases.35 These errors included inadequate supervision by an anesthesiologist and lapses in postoperative monitoring.
Physiologic and Anatomic Changes in Pregnancy
Of the multitude of anatomic and physiologic changes that occur in pregnancy (see Chapter 2), some have a significant effect on the degree of difficulty of laryngoscopy and tracheal intubation (Box 30-1).
Airway Edema
Fluid retention makes the tissues of the head and neck less compliant and may lead to narrowing of the upper airway, especially in the supine position. Consequently, nasal congestion, snoring, and voice changes all occur more frequently in advanced pregnancy.36 A 34% increase in Mallampati class IV scores15,37 from the first to the third trimester of pregnancy has been observed (see later discussion).38 Difficulty with intubation has been shown to be more than 11 times more common in women with Mallampati class IV than class I scores.28
Although changes in the airway develop gradually during pregnancy, more acute changes may be observed during labor. Mallampati class scores deteriorate during labor.39–41 Decreases in upper airway volume during labor have been demonstrated by acoustic reflectometry.39 The volume of both the oral component of the airway (from the incisors to the oropharyngeal junction) and the pharyngeal component (from the oropharyngeal junction to the glottis) are decreased, presumably as a result of increasing soft tissue edema. Airway narrowing may be more significant in women with preeclampsia. The airway edema that has been observed during labor may be exacerbated by expulsive efforts during the second stage of labor42 or after extubation after cesarean delivery.28 It is therefore prudent to reevaluate the airway before induction of general anesthesia rather than rely solely on a prelabor assessment.39
Nasal capillary engorgement during pregnancy increases the risk for epistaxis after nasal instrumentation and has led many practitioners to believe that nasal intubation is relatively contraindicated in pregnancy. In a 2011 review, Arendt et al.43 challenged this opinion, suggesting that nasal fiberoptic intubation is acceptable after careful and appropriate preparation of the nasal mucosa with topical vasoconstrictors. However, the effects of topical agents on both the prevention of epistaxis and maternal hemodynamic parameters and uteroplacental perfusion must be evaluated and the relative risk of this procedure should be assessed on an individual basis.
Respiratory and Metabolic Changes
As pregnancy progresses, the gravid uterus increasingly encroaches on the diaphragm and lung volumes are reduced. By term, expiratory reserve volume decreases by 25% and residual volume decreases by 15%, resulting in a 20% reduction in functional residual capacity (FRC). This decrease is more marked in the supine than upright position, and in the obese than lean patient. Closing volume is unchanged in pregnancy, but the decrease in FRC results in airway closure in 50% of women if they are supine.44 Metabolic requirements for oxygen increase by nearly 60% during pregnancy, predominantly because of fetal demands. Oxygen requirement is further increased during labor (see Chapter 2). These changes make pregnant women more likely to become hypoxemic during periods of apnea such as during the induction of general anesthesia.45 Therefore, adequate denitrogenation (so-called preoxygenation—replacing nitrogen in the FRC with oxygen) is vital to delay the onset of hypoxemia during periods of apnea (see later discussion).
Preoxygenation and the rate of hemoglobin desaturation have been investigated by computer modeling.46–48 In these models, labor, morbid obesity, and sepsis all hasten preoxygenation; however, desaturation also occurs more rapidly in the moderately ill and the obese (Figure 30-1). Significantly, the time to life-threatening hypoxemia is significantly shorter than that for recovery from paralysis from succinylcholine.46 Therefore, should ventilation be impossible, it cannot be assumed that the patient will recommence breathing before dangerously low levels of oxygen saturation have been reached.
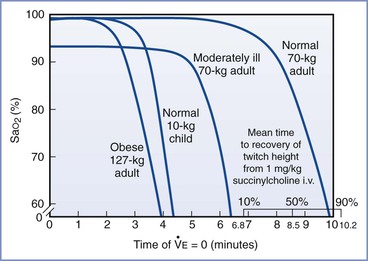
FIGURE 30-1 Time to hemoglobin desaturation (initial SaO2 = 0.87). SaO2 versus time of apnea for various types of patients. (From Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology 1997; 87:979-82.)
Weight Gain
During pregnancy, most women gain 10 to 15 kg (22 to 33 lb) or more. This weight gain is composed of increases in fat deposition, blood and interstitial fluid volume, and uterine and fetal mass. High BMI is associated with difficulty in mask ventilation and tracheal intubation4,27 and with a greater risk for requiring emergency cesarean delivery.24 BMI is directly associated with more rapid oxygen desaturation during apnea during the induction of general anesthesia.
Breast Enlargement
Breast enlargement during pregnancy may impede intubation by interfering with correct placement of the laryngoscope blade and laryngoscopic manipulation to improve visualization of the larynx. Various strategies can minimize this problem, the most important of which is optimizing the patient’s position. With both arms abducted, breast tissue falls away from the chest. Ensuring that the patient is in the ideal intubating position (discussed later) further helps with laryngoscope blade insertion; a short-handled laryngoscope is recommended. The handle can be directed toward the shoulder on insertion of the blade and then redirected once the blade is in the oropharynx.
Full Dentition
Full dentition is typically present in young pregnant women and can interfere with direct laryngoscopy, particularly if the maxillary incisors are protruding or the thyromental distance is small.28
Gastroesophageal Changes
Pregnancy-induced changes in the gastroesophageal system do not per se make laryngoscopy and intubation more difficult. However, owing to the increased risk for regurgitation and aspiration from the second trimester onward (see Chapters 2 and 29), rapid-sequence induction of anesthesia is advocated for almost all parturients, thus potentially increasing the risk for difficult airway management. Antacid prophylaxis is therefore mandatory when surgical intervention is required.
Airway Assessment
Preanesthetic assessment of the airway is necessary before both general or neuraxial anesthesia, so that plans for airway management can be made in advance. A variety of bedside tests have been used, either singularly or in combination, to predict the airway difficulty. The validity of many tests has been questioned, and it is useful to consider how these assessments have been investigated.49 First, airway difficulty, the outcome, must be defined. A number of definitions have been used (see earlier discussion), including difficulty or failure with ventilation (with or without a supraglottic airway) or intubation. Second, various predictive factors that are associated with difficult airway management have been tested on different sample populations of patients.
For an assessment to be useful it must be both sensitive (i.e., correctly identify those whose tracheas are difficult to intubate) and specific (i.e., correctly identify those whose tracheas are easy to intubate). Despite having both reasonably high sensitivity and specificity, many predictive tests have limited use in the clinical environment because failed intubation is rare; the number of false-positive tests (those predicted to be difficult that are not) will always be significantly higher than the number of true-positive tests (those predicted to be difficult that are).49 The positive predictive value (ratio of true-positive tests to the total number of positive tests) for individual difficult airway tests is typically less than 50%, that is, fewer than half of the procedures predicted to be difficult will actually be difficult.49 However, combining difficult airway tests can raise the index of suspicion for difficulty with airway management. Despite these shortcomings in difficult airway prediction, airway assessment is a vital part of anesthetic management. Preanesthetic assessment allows the consideration of potential airway problems and the creation of a stepwise plan for dealing with difficulties should they arise. Common methods of airway assessment used in clinical practice are discussed next.
Cormack and Lehane Grade
Cormack and Lehane50 devised a glottic view grading system in 1984. The purpose of the system was to grade the glottic view obtained with direct laryngoscopy as a means of training for general anesthesia in the obstetric patient. Therefore, the Cormack and Lehane grade is not a preoperative assessment tool but rather a classification method to describe the relative difficulty with subsequent tracheal intubation. The original description includes four grades of laryngoscopy (Figure 30-2):
• Grade 1: Full view of glottis
• Grade 2: Partial view of glottis or arytenoids
• Grade 3: Only epiglottis visible
Subsequent modifications have been proposed. Grade 2 may be divided into 2A (part of vocal cords visible) and 2B (only arytenoids or very posterior origin of vocal cords visible).51,52 Further, Grade 3 may be divided into those in which the epiglottis is visible and lifted, such as with a gum elastic bougie (Grade 3A), and those in which the epiglottis is visible but not able to be lifted (Grade 3B).52,53 Increasing difficulty with intubation is to be expected with each progressive grade of the Cormack and Lehane classification.
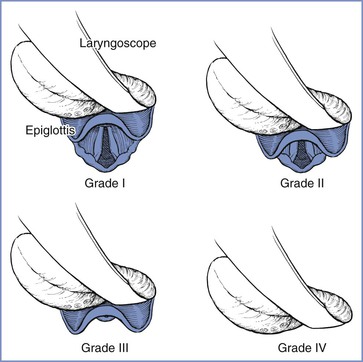
FIGURE 30-2 Cormack and Lehane laryngoscopic view grades. Grade I is visualization of the entire laryngeal aperture. Grade II is visualization of only the posterior portion of the laryngeal aperture. Grade III is visualization of only the epiglottis. Grade IV is visualization of only the soft palate. (From Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia 1984; 39:1105-11.)
Because of the widespread acceptance of the Cormack and Lehane grading system, some useful information can be gained by reviewing the anesthetic records of patients who have a previous history of direct laryngoscopy; the Cormack and Lehane glottic view grade is often documented. However, prior reports should be treated with caution because grades given in the nonpregnant state will likely differ from those determined during pregnancy, and the potential for inter-observer and intra-observer variability exists.
Mallampati Class
In 1985 Mallampati et al.37 described a three-point scale of the oropharyngeal view of the open mouth based on concealment of the faucal pillars, soft palate, and uvula by the base of the tongue; the more the view was obscured, the greater the difficulty with laryngoscopy and intubation. Samsoon and Young15 later modified the scoring system into a four-point scale (Figure 30-3):
Class I: visualization of soft palate, uvula, and tonsillar pillars
Class II: visualization of soft palate and base of uvula
Class III: visualization of soft palate only
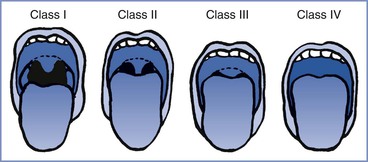
FIGURE 30-3 Modified Mallampati classification of the oropharynx. Classification of the upper airway in terms of the size of the tongue and the pharyngeal structures that are visible with the mouth open. In class I, the soft palate, uvula, and anterior and posterior tonsillar pillars can be seen. In class II, the soft palate and uvula can be seen; the tonsillar pillars are hidden by the tongue. In class III, the soft palate and the base of the uvula can be seen. In class IV, only the hard palate can be seen. (From Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 1985; 32:429-34.)
The test should be performed with the patient sitting upright with her head in the neutral position. The patient is instructed to open her mouth as wide as possible and protrude her tongue as far as possible without phonation. Increasing difficulty with laryngoscopy and tracheal intubation has been demonstrated with greater Mallampati scores in both obstetric28 and nonobstetric populations.37
Mallampati scores are frequently used as part of an assessment to predict difficult intubation. It is important to remember that scores change during pregnancy38 and during labor.39–41 When used as the sole predictor of difficult airway, the incidence of both significant false-positive and false-negative results is high.54 This poor predictive value may be explained by the use of phonation, poor patient positioning, involuntary arching of the tongue, and interobserver variability in interpretation. A meta-analysis of the Mallampati score concluded that the test had limited accuracy for predicting a difficult airway and was not a useful screening test.55 Consequently, the Mallampati score is best used in combination with other tests.
Thyromental Distance
During laryngoscopy, the tongue is normally pushed into the mandibular space. The thyromental distance, the distance from the tip of the chin to the notch of the thyroid cartilage, can be used to estimate the size of this space and therefore whether the tongue can easily be displaced to facilitate laryngoscopy.56 In the absence of other abnormalities, if the thyromental distance is more than 6.5 cm and the horizontal mandibular length more the 9 cm, intubation should proceed without difficulty. A thyromental distance of less than 6 cm suggests an increased risk for difficulty.54 However, lack of detail in various studies regarding precisely how the thyromental distance was measured (whether it was performed from the inner or outer border of the mandible) make interpretation of this test difficult.
Anatomically, if the mandibular space is small and unable to accommodate the tissues displaced by the laryngoscope blade, few alterations will improve the line of vision during direct laryngoscopy (Figure 30-4).56 When the mandibular space is small, the larynx lies relatively anterior and the tongue must be pulled forward maximally and compressed to expose the larynx.
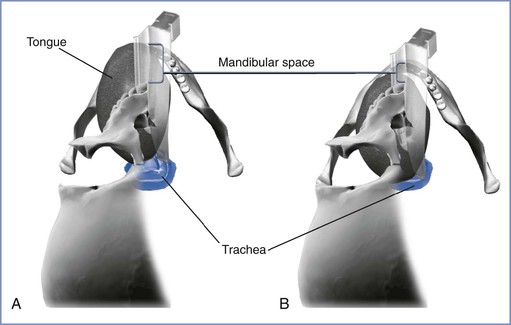
FIGURE 30-4 The mandibular space viewed by the laryngoscopist inserting a curved laryngoscope blade into the airway in a supine patient. The mandibular space is the area bounded by the plane of the line of vision and the part of the mandibular arch in front of this plane (lower and upper extent of brackets, respectively). A, Normal-size mandibular space with room for the tongue. The laryngoscopist has an unimpeded view of the glottis. B, Small mandibular space—the tongue impedes the view of the glottis. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Atlanto-occipital Joint Extension
Extension of the atlanto-occipital joint is necessary for the patient to be in the ideal intubating position in which the oral, pharyngeal, and laryngeal axes are aligned (see later discussion). Movement can be assessed with the patient seated with the head and neck in the neutral position facing forward and then with the joint maximally extended (Figure 30-5). Normal extension should be 35 degrees or more; difficulty with intubation can be expected when joint movement is decreased.56 The accuracy of this assessment is subject to inter-observer variability, making its role in routine airway assessment questionable.
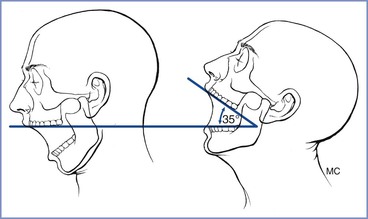
FIGURE 30-5 Clinical method for quantifying atlanto-occipital joint extension. When the head is held erect and faces forward, the plane of the occlusal surface of the upper teeth is horizontal and parallel to the floor. When the atlanto-occipital joint is extended, the occlusal surface of the upper teeth form an angle with the plane parallel to the floor. The angle between the erect and the extended planes of the occlusal surface of the upper teeth quantifies the atlanto-occipital joint extension. A normal person can produce 35 degrees of atlanto-occipital joint extension. (From Bellhouse CP, Dore C. Criteria for estimating likelihood of difficulty of endotracheal intubation with Macintosh laryngoscope. Anaesth Intensive Care 1988; 16:329-37.)
Mandibular Protrusion
The patient’s ability to extend the mandibular teeth anteriorly beyond the line of the maxillary teeth may predict adequate visualization of the larynx during direct laryngoscopy. In the mandibular protrusion test, patients are asked to protrude their mandible as far as possible (Figure 30-6); one of three classes is assigned57,58:
• Class A: The lower incisors can protrude anterior to the upper incisors.
• Class B: The lower incisors can be brought edge to edge with the upper incisors.
• Class C: The lower incisors cannot be brought edge to edge with the upper incisors.
Class A is a good predictor of a good glottic view with direct laryngoscopy whereas class C is associated with poor glottic view.57
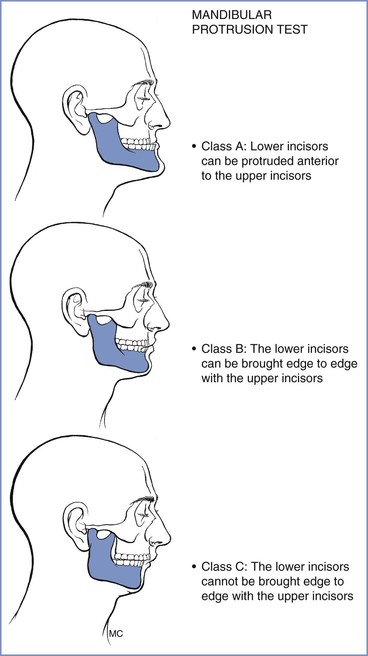
FIGURE 30-6 Mandibular protrusion test. Three classifications are based on the test, which is also referred to as the upper lip bite test. (Redrawn from Munnur U, de Boisblanc B, Suresh MS. Airway problems in pregnancy. Crit Care Med 2005; 33:S259-68.)
The upper lip bite test (ULBT) is similar to mandibular protrusion. In class 1 the lower incisors can bite the upper lip above the vermillion border (i.e., the normally sharp demarcation between the lip and the adjacent normal skin); in class 2, the lower incisors can bite the upper lip below the vermillion border; and in class 3, the lower incisors cannot bite the upper lip.59 The ULBT has been shown to be a better predictor than a Mallampati score for predicting ease with laryngoscopy and intubation.59 The ULBT cannot be assessed in edentulous patients.
Other Assessments
Sternomental distance has been suggested to predict difficult laryngoscopy. This distance is measured between the chin and sternum with the head fully extended on the neck and the mouth closed. Unfortunately, the assessment has extremely weak predictive power, and consequently it has largely been abandoned.
Limited mouth opening impedes the introduction of laryngoscope blade as well as other airway devices; an interincisor distance of less than 5 cm may predict difficult intubation. Mouth opening of less than two fingerbreadths has been shown to reduce the prevalence of easy intubation from 95% to 62%.60 Mouth opening also is influenced by cervical spine movement; if movement is limited, mouth opening may also be restricted.61 Protruding maxillary incisors, a single maxillary incisor, and missing maxillary incisors have been shown to be predictive of difficult intubation in obstetric patients.28
Comorbidities, including those not related to pregnancy, may influence airway management and should be considered before anticipated airway management. Most notably, maternal obesity is associated with an increased incidence of airway problems (see earlier discussion).24,25,27 Similarly, difficulties in airway management should be anticipated in patients with severe preeclampsia.
Multivariable Assessments
Individual tests are poorly predictive of airway difficulty; therefore, a number of investigators have combined assessments in an effort to improve specificity. Wilson et al.62 assessed five risk factors (weight, head and neck movement, jaw movement, presence or absence of a receding mandible, prominent teeth). Each variable was scored from 0 to 2, giving a Wilson risk sum. Although 75% of cases of difficult laryngoscopy could be predicted, 12% were falsely predicted to be difficult.62 Subsequent work using the Wilson risk sum found a positive predictive value of only 9%, and consequently it is now rarely used in clinical practice.63
Frerk64 demonstrated that a combination of the Mallampati score and thyromental distance was more predictive than either test alone; the combined assessment had a sensitivity of 81% and a specificity of 98% in predicting a difficult airway. However, owing to the rarity of difficult intubation, the positive predictive value was only 64%.64 Realizing that there was an absence of a clear description and agreement as to the method of performing individual tests, Lewis et al.65 assessed different methods of grading the oropharyngeal view and the mandibular space as predictors of difficult laryngoscopy. Twenty-four different oropharyngeal assessments were considered using two body positions, three head positions, and two tongue positions, each with and without phonation. Similarly, the mandibular space was measured in 24 ways using two body positions, three head positions, and two distal and two proximal endpoints. The results were subject to logistic regression analysis. Although most difficult intubations could be predicted, half of those that were anticipated to be difficult were ultimately found to be easy, even with the most predictive combination of tests.65
Tse et al.66 combined the angle at full head extension (in an upright position, the angle made by a line joining the ear tragus [apex] and the corner of the mouth to a line parallel to the floor [horizontal]), thyromental distance, and Mallampati classification in an attempt to predict difficult intubation in a general surgery population. Although these tests were likely to identify easy intubations, they had low sensitivity for predicting those in whom intubation was difficult.66
In a study of 400 pregnant women scheduled for elective cesarean delivery, Honarmand and Safavi67 evaluated Mallampati class score, ratio of height to thyromental distance, and the ULBT, both in isolation and combination. A total of 8.75% patients had a Cormack and Lehane grade 3 or 4 laryngoscopic view; the ratio of height to thyromental distance was the best predictor of this outcome.67
Recommendations
The thoroughness of the airway assessment often depends on the urgency with which surgery needs to be performed. For emergency procedures, relatively little time is available; and so it is prudent to assess all women in the labor and delivery suite soon after their arrival, focusing on those with the greatest risk for intervention.68 However, changes in assessment during the course of labor must be anticipated, and reevaluation before inducing anesthesia is vital to the safe care of these patients.
The assessment should attempt to identify the patients who will be difficult to ventilate and whose tracheas will be difficult to intubate. It should start with a history to detect factors that may indicate the presence of a difficult airway, as well as the potential risk for pulmonary aspiration. Examination of previous anesthetic records, if available, may indicate problems with ventilation or intubation. The presence of comorbidities such as obesity and preeclampsia should be considered. The ASA Practice Guidelines for Management of the Difficult Airway2 list 11 components that can be assessed (Table 30-3), acknowledging the absence of a single test that can reliably predict who is likely to present difficulty with airway management. Consequently, a combination of assessments is generally considered preferable.
TABLE 30-3
Components of Preoperative Airway Examination
| Airway Examination Component | Nonreassuring Findings |
| 1. Length of upper incisors | Relatively long |
| 2. Relation of maxillary and mandibular incisors during normal jaw closure | Prominent overbite (maxillary incisors anterior to mandibular incisors) |
| 3. Relation of maxillary and mandibular incisors during voluntary protrusion | Patient cannot bring mandibular incisors anterior to maxillary incisors |
| 4. Interincisor distance | Less than 3 cm |
| 5. Visibility of uvula | Not visible when tongue protruded with patient sitting (e.g., Mallampati class > II) |
| 6. Shape of palate | Highly arched or very narrow |
| 7. Compliance of mandibular space | Stiff, indurated, occupied by mass or nonresilient |
| 8. Thyromental distance | Less than three ordinary fingerbreadths |
| 9. Length of neck | Short |
| 10. Thickness of neck | Thick |
| 11. Range of motion of head and neck | Patient cannot touch tip of chin to chest or cannot extend neck |
Modified from American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway. Anesthesiology 2013; 118:251-70.
Performing and documenting mouth opening, the Mallampati class, atlanto-occipital mobility, thyromental distance, and mandibular protrusion may be performed relatively quickly and should identify most patients who will present difficulties with airway management. The preanesthesia evaluation should seek to identify risk factors for difficulty with mask ventilation, laryngoscopy, airway device insertion (including intubation), and performance of a surgical airway. When risk factors are identified, appropriate plans for airway management, such as the ready availability of additional equipment and personnel (e.g., individuals experienced with airway management and the creation of a surgical airway) should be made. The proposed plan should consider that the administration of a neuraxial anesthetic technique may be the safest option for both mother and infant, even in the presence of nonreassuring fetal status. The plan must also include alternatives for situations in which the initial plan is not possible. The risks and benefits of various alternatives should be discussed with the patient and the obstetric, neonatal, and nursing teams and documented in the patient’s medical record.
Prophylaxis
Neuraxial Labor Analgesia
The widespread acceptance and use of neuraxial analgesic and anesthetic techniques for obstetric patients has significantly reduced the need for general anesthesia and airway manipulation. In obstetric patients in whom difficulty in airway management or neuraxial technique administration is anticipated or when risk factors for an urgent or emergent cesarean delivery are present, early or prophylactic placement of an epidural catheter should be encouraged. A prophylactic epidural catheter is one that is placed and tested with a small dose of local anesthetic; analgesia is not established until active labor begins, the patient requests analgesia, and/or an operative delivery is required. Such a catheter provides a readily available conduit for providing neuraxial analgesia or anesthesia, especially if rapid onset (e.g., an emergency cesarean delivery) is desirable. Early epidural catheter placement also allows the procedure to take place in a controlled setting and allows time for catheter manipulation and replacement, if necessary, before further pathophysiologic changes (e.g., decreased platelet count, worsening airway edema) occur. The correct placement of the epidural catheter in the epidural space should be tested with the injection of a local anesthetic test dose and careful bilateral sensory testing to confirm the presence of bilateral neuroblockade.
Unfortunately, labor analgesia cannot always be successfully converted to surgical anesthesia for an operative delivery; reported failure rates are as high as 8%.69 A 2012 meta-analysis has demonstrated the need for conversion to general anesthesia in 5% of women who receive epidural analgesia in labor70; higher failure rates are observed among women requiring more physician interventions for inadequate epidural labor analgesia, in settings of need for urgent delivery, and when an anesthesiologist without specialty training or experience in obstetric anesthesia is providing care. Consequently, women receiving labor epidural analgesia must be evaluated at regular intervals; if analgesia is inadequate, re-siting the epidural catheter must be considered. A meta-analysis of studies comparing different local anesthetics for conversion of epidural analgesia to anesthesia found that lidocaine with epinephrine has a faster onset than bupivacaine, levobupivacaine, or ropivacaine.71 The addition of bicarbonate to chloroprocaine or lidocaine with epinephrine further hastens the onset of local anesthetic action (see Chapter 26).
In situations in which conversion of epidural analgesia to anesthesia is not possible, general anesthesia may still be avoided if time permits the initiation of spinal or combined spinal-epidural anesthesia. However, care should be taken when performing a spinal anesthetic after a failed epidural top-up dose of local anesthetic, because cases of high and total spinal anesthesia have been reported in this setting (see Chapter 26). An airway management plan must always be in place, even if the primary plan is for the administration of neuraxial anesthesia.
Fasting and Antacid Prophylaxis
All obstetric patients requiring surgical anesthesia are at risk for pulmonary aspiration of gastric contents, particularly if airway difficulties are encountered (see Chapter 29). Because conversion from neuraxial to general anesthesia may be required either before or during surgery, strategies must be adopted to minimize this risk. The ASA and the American College of Obstetricians and Gynecologists (ACOG) recommendations allow modest amounts of clear liquid with uncomplicated labor, but they recommend the avoidance of solid foods in laboring women.72,73 Clear liquids and solids are allowed up to 2 hours and 6 to 8 hours, respectively, before an elective operative procedure.72,73 However, more liberal policies on oral intake in labor have become increasingly widespread as maternal death from aspiration becomes less common. The U.K. National Institute for Health and Clinical Excellence suggests that women be allowed to drink isotonic fluids in labor and eat a light diet unless they develop risk factors that make general anesthesia more likely.74 Consequently, all laboring women should be assessed and oral intake restricted if surgical intervention appears likely.
Once surgical intervention is required, antacids such as intravenous histamine-2 (H2)-receptor antagonists or proton-pump inhibitors should be administered in case general anesthesia is necessary. However, these drugs take up to 30 minutes to become effective. If emergency general anesthesia is required, oral administration of a nonparticulate antacid such as sodium citrate is used to increase the pH of gastric contents. A dose of sodium citrate (0.3 molar) 30 mL is effective for approximately 30 minutes and should be administered shortly before the induction of general anesthesia.75
Metoclopramide promotes gastric emptying and increases lower esophageal sphincter tone, although its efficacy is decreased by concurrent use of opioids. It may be given orally in labor or intravenously before general anesthesia.
A number of deaths from presumed aspiration after tracheal extubation were reported in the most recent Confidential Enquiries into Maternal Deaths in the United Kingdom report.6 The report’s authors recommended that when general anesthesia is administered to woman with a potentially full stomach, consideration should be given to passing an “in and out” orogastric tube before extubation.
Patient Positioning
The optimal view during laryngoscopy, which yields the best chance for a successful intubation, requires appropriate patient positioning. The sniffing position, with 35 degrees of neck flexion and 15 degrees of head extension, has been considered the ideal position for facilitating a view of the glottis by aligning the oral, pharyngeal, and laryngeal axes (Figure 30-7).76 Although use of the sniffing position has recently been questioned, most studies find it superior to other positions. The correct use of the sniffing position requires that the external auditory meatus and sternum are in horizontal alignment. Some video laryngoscopes (see later discussion) do not require the patient’s head and neck to be in the sniffing position for successful device use.
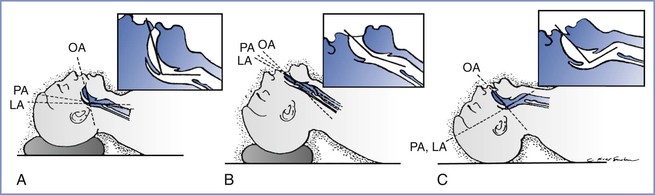
FIGURE 30-7 Head and neck position during laryngoscopy. As the head position changes from neutral, the alignment of the oral axis (OA), pharyngeal axis (PA), and laryngeal axis (LA) changes within the upper airway. A, The head is resting on a large pad that flexes the neck on the chest and aligns the LA with the PA (the neutral position). B, The head is resting on a pad (which flexes the neck on the chest) and concomitant extension of the head on the neck can be seen, which brings all three axes into alignment (the sniffing position). C, Extension of the head on the neck without concomitant elevation of the head on a pad, which results in nonalignment of the PA and LA with the OA. (From Benumof JL. Conventional [laryngoscopic] orotracheal and nasotracheal intubation [single-lumen type]. In Benumof JL, editor. Clinical Procedures in Anesthesia and Intensive Care. Philadelphia, JB Lippincott, 1991:115-48.)
For obese patients, a ramped position is preferable (Figure 30-8). The anteroposterior chest diameter is increased in obese patients, making 35 degrees of neck flexion unachievable in the supine position. Consequently, the shoulders and upper torso need to be raised; this position can be achieved with the use of blankets or pillows or one of the many commercially available, wedge-shaped positioning cushions. Optimal elevation is verified by checking that the external auditory meatus and sternoclavicular joint are in horizontal alignment. Elevating the back of the operating table by 25 degrees may make laryngoscopy easier and also aids preoxygenation (discussed later). The operating room table should be elevated to a height at which the laryngoscopist is most comfortable, with space at the head of the bed to accommodate access for the anesthesia team and necessary equipment.
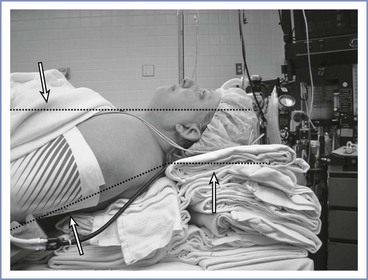
FIGURE 30-8 A morbidly obese patient is in an optimal position for direct laryngoscopy when an imaginary horizontal line can be drawn from the sternal notch through (or slightly anterior to) the external auditory meatus. To achieve this, the upper back and shoulders should be significantly elevated with pads or blankets (or commercial elevation wedge/pillow) to allow the head to be extended at the atlanto-occipital joint. Additional blankets should be used to support the head in this position.
Left uterine displacement to minimize aortocaval compression should be maintained during preparation for, and induction of, general anesthesia. This may be achieved by tilting the operating table or by placing a wedge under the right hip.
Denitrogenation (Preoxygenation)
In pregnancy, the decrease in FRC and increase in oxygen requirement result in rapid oxygen desaturation during periods of apnea (e.g., during induction of general anesthesia). The FRC is the primary reservoir for oxygen during apnea. Therefore, effective denitrogenation, or preoxygenation, of the FRC is vital to delay the onset of hypoxemia.
The standard technique for preoxygenation has been to breathe 100% oxygen through a tight-fitting facemask at normal tidal volumes for 3 to 5 minutes. Given the urgent nature of obstetric general anesthesia, attention has focused on whether several maximal deep breaths over a shorter period can be as effective. Chiron et al.77 compared a traditional 3-minute technique with either eight deep breaths over 1 minute (8 DB/1 min) or four deep breaths over 30 seconds (4 DB/30 sec). By monitoring with end-tidal fractional oxygen concentration (FETO2), which is probably the best marker of lung denitrogenation, the authors found that 3 minutes of tidal volumes or the 8 DB/1 min technique was more effective than the 4 DB/30 sec technique. They suggested using the 8 DB/1 min technique in the setting of emergency obstetric anesthesia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree