 |
 |
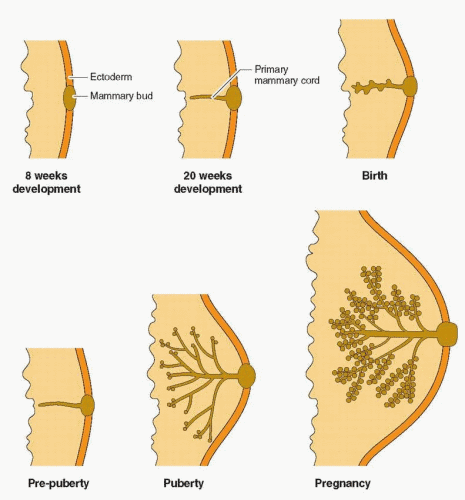
of the lobule that later become the milk-producing structures.2 However, neither hormone alone, or in combination, is capable of yielding optimal breast growth and development. Full differentiation of the gland requires insulin, cortisol, thyroxine, prolactin, and especially, growth hormone-induced insulin-like growth factor-I.3,4 Experimental evidence in mice knock-out models supports the combined actions of estrogen and progesterone, mediated primarily by estrogen receptor-a and progesterone receptor-B, but dependent on epidermal growth factor and IGF-I.5,6 and 7 Estrogen and progesterone receptors in normal breast tissue are located in non-dividing epithelial cells and in stromal cells adjacent to proliferating epithelial cells, indicating the importance of paracrine communication using growth factors. Growth hormone-induced IGF-I is essential in both mammary development and function.4
mitotic activity, and DNA production of nonglandular tissue and glandular epithelium peak during the luteal phase.9,10 and 11 This accounts for cystic and tender premenstrual changes.
 |
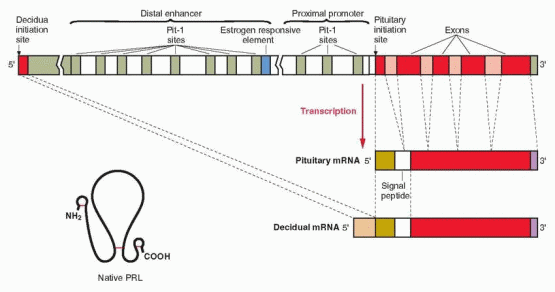
secretion is exerted by estrogen, producing both differentiation of lactotrophs and direct stimulation of prolactin production.32,33 An estrogen response element is adjacent to one of the Pit-1 binding sites in the distal enhancer region, and estrogen stimulation of the prolactin gene involves interaction with this Pit-1 binding site. Estrogen additionally influences prolactin production by suppressing dopamine secretion.34 Prolactin is also synthesized in extrapituitary tissues, including breast tissue and endometrial decidua.35 In extrapituitary sites, the active promoter site is upstream of the pituitary initiation site, and is not regulated by Pit-1, estrogens, or dopamine. Progesterone increases prolactin secretion in the decidua, but has no effect in the pituitary.
 |
exists in a long form and a short form, but only the D2 (long form) is present on lactotrophs. The molecular mechanism for dopamine’s inhibitory action is still not known. There are several other PIFs, but a specific role has been established only for dopamine.
Maternal prolactin does not pass to the fetus in significant amounts. Indeed, the source of amniotic fluid prolactin is neither the maternal pituitary nor the fetal pituitary. The failure of dopamine agonist treatment to suppress amniotic fluid prolactin levels, and studies with in vitro culture systems indicate a primary decidual source with transfer via amnion receptors to the amniotic fluid, requiring the intactness of amnion, chorion, and adherent decidua. This decidual synthesis of prolactin is initiated by progesterone, but once decidualization is established, prolactin secretion continues in the absence of both progesterone and estradiol.42 Various decidual factors regulate prolactin synthesis and release, including relaxin, insulin, and insulin-like growth factor-I. Prolactin produced in extrapituitary sites involves an alternative exon upstream of the pituitary start site, generating a slightly larger RNA transcript compared with the pituitary product. However, the amino acid sequence and the chemical and biologic properties of decidual prolactin are identical to those of pituitary prolactin. It is hypothesized that amniotic fluid prolactin plays a role in modulating electrolyte economy not unlike its ability to regulate sodium transport and water movement across the gills in fish (allowing the ocean-dwelling salmon and steelhead to return to freshwater streams for reproduction). Thus prolactin would protect the human fetus from dehydration by control of salt and water transport across the amnion. Prolactin reduces the permeability of the human amnion in the fetal to maternal direction by a receptor-mediated action on the epithelium lining the fetal surface.43 Decidual and amniotic fluid prolactin levels are lower in hypertensive pregnancies and in patients with polyhydramnios.44,45 Prolactin receptors are present in the chorion laeve, and their concentration is lower in patients with polyhydramnios.46 Thus, idiopathic polyhydramnios may be a consequence of impaired prolactin regulation of amniotic fluid.
 |
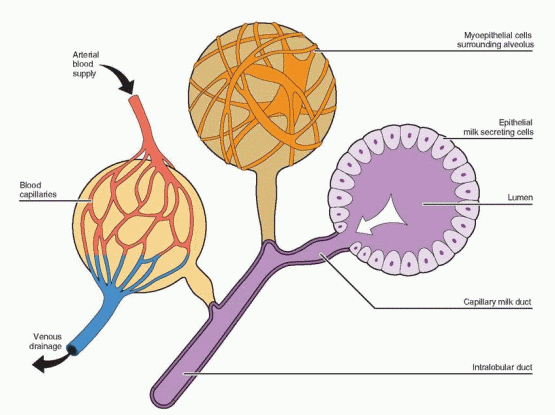
the paraventricular and supraoptic nuclei of the hypothalamus to synthesize and transport oxytocin to the posterior pituitary. The efferent arc (oxytocin) is blood-borne to the breast alveolus-ductal systems to contract myoepithelial cells and empty the alveolar lumen. Milk contained in major ductal repositories is ejected from 15 to 20 openings in the nipple. This rapid release of milk is called “let-down.” This important role for oxytocin is evident in knockout mice lacking oxytocin who undergo normal parturition, but fail to nurse their offspring.80 The milk ejection reflex involving oxytocin is present in all species of mammals. Oxytocin-like peptides exist in fish, reptiles, and birds, and a role for oxytocin in maternal behavior may have existed before lactation evolved.78
Rule of 3’S for Postpartum Initiation of Contraception | ||||
|---|---|---|---|---|
|
Increased prolactin release can be a consequence of prolactin elaboration and secretion from pituitary tumors (discussed in Chapter 11), which function independently of the otherwise appropriate restraints exerted by PIF from a normally functioning hypothalamus. This infrequent but potentially dangerous tumor, which has endocrine, neurologic, and ophthalmologic liabilities that can be disabling, makes the differential diagnosis of persistent galactorrhea a major clinical challenge. Beyond producing prolactin, the tumor may also suppress pituitary parenchyma by expansion and compression, interfering with the secretion of other tropic hormones. Other pituitary tumors may be associated with lactotroph hyperplasia and present with the characteristic syndrome of hyperprolactinemia and amenorrhea.
A variety of drugs can inhibit hypothalamic dopamine.97 There are nearly 100 phenothiazine derivatives with indirect mammotropic activity. In addition, there are many phenothiazine-like compounds, reserpine derivatives, amphetamines, and an unknown variety of other drugs (opiates, diazepams, butyrophenones, verapamil, a-methyldopa, and tricyclic antidepressants) that can initiate galactorrhea via hypothalamic suppression. The final action of these compounds is either to deplete dopamine levels or to block dopamine receptors. Chemical features common to many of these drugs are an aromatic ring with a polar substituent as in estrogen and at least two additional rings or structural attributes making spatial arrangements similar to estrogen. Thus, these compounds may act in a manner similar to estrogens to decrease PIF or to act directly on the pituitary. In support of this conclusion, it has been demonstrated that estrogen and phenothiazine derivatives compete for the same receptors in the median eminence. Prolactin is uniformly elevated in patients on therapeutic amounts of these drugs, but essentially never as high as 100 ng/mL. Approximately 30-50% exhibit galactorrhea that should not persist beyond 3-6 months after drug treatment is discontinued.
Hypothyroidism (juvenile or adult) can be associated with galactorrhea. With diminished circulating levels of thyroid hormone, hypothalamic TRH is produced in excess and acts as a PRF to release prolactin from the pituitary. Reversal with thyroid hormone is strong circumstantial evidence to support the conclusion that TRH stimulates prolactin.
Excessive estrogen (e.g., oral contraceptives) can lead to milk secretion via hypothalamic suppression, causing reduction of dopamine and release of pituitary prolactin, and direct stimulation of the pituitary lactotrophs. Galactorrhea developing during oral contraceptive administration may be most noticeable in the traditional dosing regimen during the 7 days free of medication (when the steroids are cleared from the body and the prolactin interfering action of the estrogen and progestin on the breast wanes). Galactorrhea caused by excessive estrogen disappears within 3-6 months after discontinuing medication. This is now a rare occurrence with the lower-dose pills.98 A longitudinal study of 126 women did demonstrate a 22% increase in prolactin values over mean control levels, but the response to low-dose oral contraceptives was not out of the normal range.99
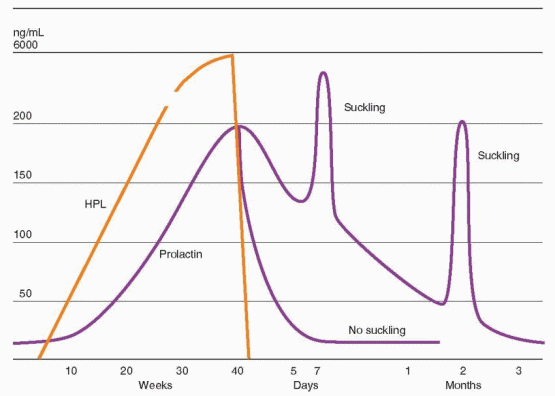
Prolonged intensive suckling can also release prolactin, via hypothalamic reduction of dopamine. Similarly, thoracotomy scars, cervical spinal lesions, and herpes
zoster can induce prolactin release by activating the afferent sensory neural arc, thereby simulating suckling. Galactorrhea and elevated prolactin levels have been observed secondary to nipple piercing.100
Stresses can inhibit hypothalamic dopamine, thereby inducing prolactin secretion and galactorrhea. Trauma, surgical procedures, and anesthesia can be seen in temporal relation to the onset of galactorrhea.
Hypothalamic lesions, stalk lesions, or stalk compression (events that physically reduce production or delivery of dopamine to the pituitary) allow release of excess prolactin leading to galactorrhea.
Increased prolactin concentrations can result from nonpituitary sources such as lung, ovarian, and renal tumors and even a uterine leiomyoma. Severe renal disease requiring hemodialysis is associated with elevated prolactin levels due to the decreased glomerular filtration rate.
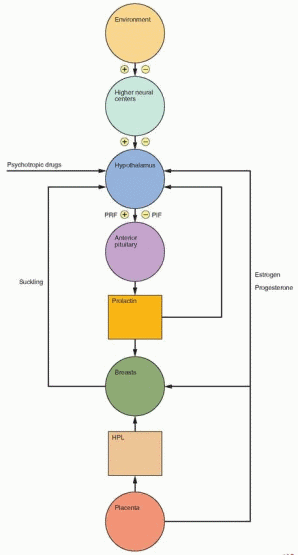 |
capable of interacting with breast prolactin receptors. On the other hand, galactorrhea can be seen in women with normal prolactin serum concentrations. Episodic fluctuations and sleep increments may account for this clinical discordance, or, in this case, bioactive prolactin may be present that is immunoreactively not detectable. Remember that at any one point in time, the bioactivity (galactorrhea) and the immunoreactivity (immunoassay result) of prolactin represent the cumulative effect of the family of structural and molecular prolactin variants present in the circulation.
effective for treating mammary discomfort and benign disease.111,112 and 113 In a comparison study, tamoxifen was more effective than danazol.111
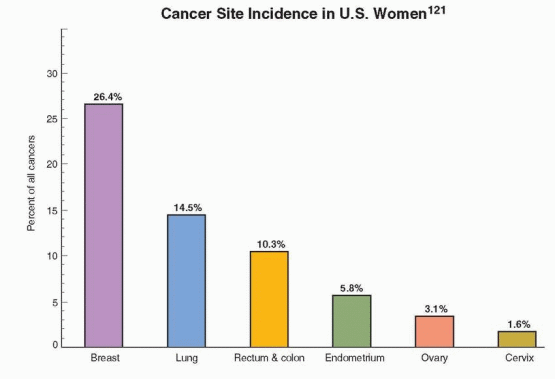 |
This decrease is believed to reflect a reduction in the use of postmenopausal hormone therapy following the publicized results of the Women’s Health Initiative and a decrease in the utilization of mammography; discussed in Chapter 18. About 87% of all breast cancers in the U.S. occur in women over age 44; only 1.9% of all cases occur under age 35, 12.5% under age 45, and 97% of breast cancer deaths in the U.S. occur in women over age 40.122
The Chances of Developing Breast Cancer in the U.S. according to Age124 | ||||||||
|---|---|---|---|---|---|---|---|---|
|
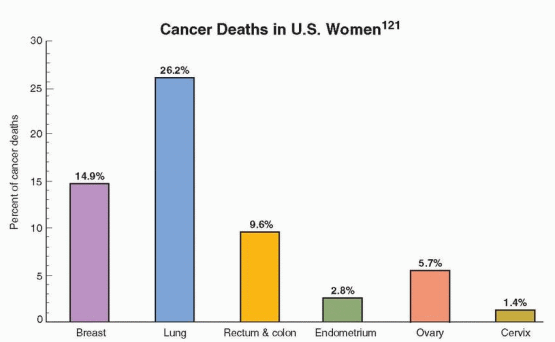 |
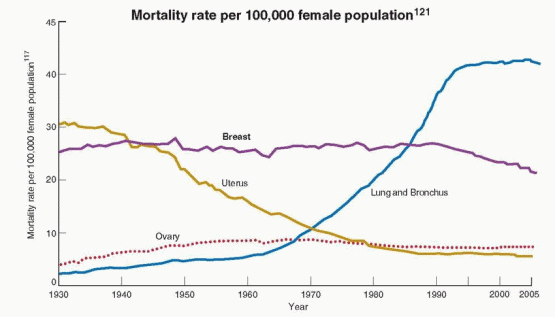 |
Risk Factors for Breast Cancer121 | ||||||
|---|---|---|---|---|---|---|
|
risk reduction in women who have oophorectomy before age 35. There is a small decrease in risk with late menarche and a moderate increase in risk with late natural menopause, indicating that ovarian activity plays a continuing role throughout reproductive life.175
The College of American Pathologists supports this position and has offered this classification.183 | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Relative Risk with Affected First-degree Relatives184 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Family History Characteristics Associated with the Presence of BRCA Mutations | ||||||
|---|---|---|---|---|---|---|
|
Affected Relative | Age of Affected Relative | Cumulative Breast Cancer Risk by Age 80 |
One first-degree relative | <50 years old 50 or more years old | 13-21% 9-11% |
One second-degree relative | <50 years old 50 or more years old | 10-14% 8-9% |
Two first-degree relatives | Both <50 years old Both 50 years or older | 35-48% 11-24% |
Two second-degree relativtes but both paternal or maternal | Both <50 years old Both 50 years or older | 21-26% 9-16% |
Summary of Breast and Ovarian Cancer Risk in BRCA Carriers201 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
6 months and annual mammography beginning at age 25. An annual evaluation by magnetic resonance imaging is also recommended because there is some evidence of a higher false-negative rate with mammography in these patients, and breast cancers detected in BRCA mutation carriers who undergo annual MRI surveillance are of lower stage disease.228 Clinical evaluation every 6 months is appropriate because the BRCA1related tumors have been demonstrated to be faster growing tumors. Support should be provided for those women who choose prophylactic mastectomy. Pelvic examination, serum CA-125 levels, and transvaginal ultrasonography with color Doppler are recommended annually for women under age 40, although it has not been demonstrated that this screening will detect tumors early enough to influence prognosis. Prophylactic salpingo-oophorectomy and hysterectomy are recommended at the completion of childbearing, preferably before age 35 and certainly by age 40. In our view, estrogen-only therapy is appropriate and acceptable following surgery, as discussed below.
reduced the risk of breast cancer by 62% in BRCA2 carriers, but had no impact in BRCA1 carriers.240,241 This is consistent with the fact that women with BRCA2 mutations have predominately estrogen receptor-positive tumors and women with BRCA1 mutations have mostly estrogen receptor-negative tumors. Although no data are available, it is likely that raloxifene and aromatase inhibitors would yield results similar to those with tamoxifen. Given the side effects associated with these drugs, the decision to use one of these agents for chemoprevention is a difficult one for both clinician and patient. Prophylactic bilateral salpingo-oophorectomy remains as the superior choice for risk protection, a procedure that can in most cases, even with thorough inspection of peritoneal surfaces and peritoneal washings, be easily performed by laparoscopy. Serial sectioning of the ovaries and tubes is mandatory to detect microscopic cancers. Although concurrent hysterectomy is an individual choice, it is recommended to gain the theoretical advantage of removing the cornual portions of the fallopian tubes.
is a correlation between intraabdominal fat (android obesity) and the risk of breast cancer, a consequence of excessive caloric consumption, however, not a specific dietary component.254 Presumably, the connection between android obesity and breast cancer is through the metabolic perturbations, especially hyperinsulinemia, associated with excessive body weight.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


