PREPARATION OF BONE MARROW–DERIVED MESENCHYMAL STEM CELLS TECHNIQUE
Harvesting
Autologous MSCs are harvested from the bone marrow of the iliac crest. This may be done under local or general anesthesia in the operating theatre and is done in the same setting as any concomitant surgery such as microfracture or realignment.
A stab incision is made over the anterior iliac crest and 60 mL of marrow is aspirated using a Jamshidi needle into a heparinized tube (Fig. 27.2). The bone marrow aspirate is processed within 60 minutes. Seventy milliliters of uncoagulated whole blood is also collected from which autologous serum is prepared and stored at −20°C.
Culture
Culture of autologous MSCs from the harvested bone marrow is carried out in a class B clean room environment. Marrow aspirate contains a mixture of different red blood cells, platelets, and leukocytes. MSC and MSC precursors compose only less than 0.05% of low-density mononuclear cells,35 and thus the purpose of culture is to generate MSCs of sufficient concentration (Fig. 27.3).
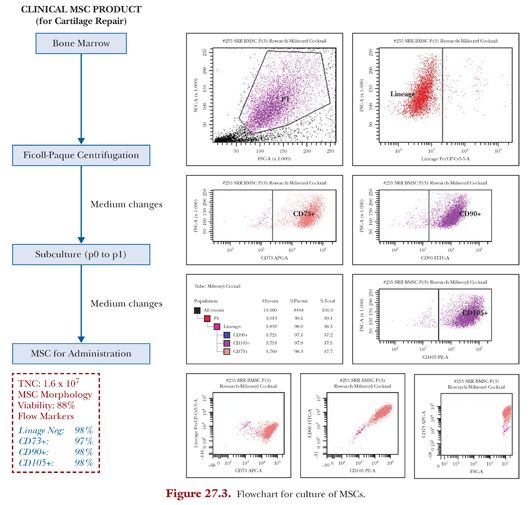
The heparinized bone marrow aspirate is mixed with a one-fifth volume of 6% (w/v) dextran (molecular weight 100,000) (Sigma-Aldrich, St. Louis, MO) and left standing at room temperature for 30 minutes to eliminate erythrocytes. The remaining leukocyte-rich fraction is washed and cultured for MSCs. These cells are cultured in 5 to 6 T75 cm2 flasks with an initial culture medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum, 50 mg/mL L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate (all from Invitrogen), and 1% antibiotic-antimycotic (penicillin 100 U/mL, streptomycin 0.1 mg/mL, amphotericin B 0.25 mg/mL) (Sigma) in a humidified atmosphere of 5% CO2 at 37°C. Cells are seeded at a density of 10,000 cells/cm2. After adherent cells are recognized, at days 5 to 7, the culture media is changed to that without antibiotics and subsequently changed every 2 to 3 days until harvest.
Sheets of cells for open surgery are formed in the presence of ascorbic acid (passage 1), whereas for injectable BMSCs, cells undergo passaging (p0 to p1) when they reach greater than 30% confluency (usually after 10 to 13 days) by treating with trypsin (Invitrogen) and washes.
Autologous serum is prepared from 70 mL of uncoagulated whole blood previously collected. The sample is centrifuged with slow acceleration; the serum is carefully aspirated and transferred to a new tube. Repeated centrifugation is performed with slow acceleration for 3 minutes at 3,000 rpm at ambient temperature.
Between 9 and 11 days, culture supernatant is sent for assays for mycoplasma and microbial contamination (aerobic and anaerobic bacteria as well as fungus).
The autologous serum is aspirated into a syringe; filtered with a sterile 0.2-mm filter and tested for sterility, anti-HIV, and hepatitis B antigen; and stored at a temperature of 20°C.
Total nucleated cell (TNC) count and viability is performed at days 9 to 12. Microscopic examination and changing of culture medium is continued regularly every 2 to 3 days till the cells are ready for harvest.
MSCs are trypsinized with collagenase and washed. A small fraction from each sample is removed to assess the cell count, viability, and flow cytometry. MSCs are ready for collection at greater than 70% confluence (estimated number of cells at time of harvest is 1.5 to 3 × 106/T75).
Viability is determined by fluorescent acridine orange, and propidium iodide staining and flow cytometry (MSC phenotyping kit, Miltenyi Biotec, Bergisch Gladbach, Germany) using FACSCanto II with FACSDiva software (BD, Franklin Lakes, NJ) is performed to stain for MSC markers—CD90+, CD105+, CD14, and CD3—to confirm that the cultured cells are MSCs.
MSC release criteria for administration include the following: no growth on mycoplasma and microbial culture, confluency greater than 70%, normal MSC morphology, and greater than 75% viable.
For direct implantation of cultured MSCs into the chondral defect, at least four sheets of cells are prepared with approximately 2 million cells/cm2 (at least 10 to 15 million cells with 96% viability). Cell sheets are transported within the patient’s serum to the operating theatre. During the arthrotomy, a periosteal patch from the proximal tibia or distal femur is harvested according to the measured size of the débrided chondral defect. The harvested periosteal patch is sutured precisely to the rim of defect, and BMSCs are implanted beneath the patch. Microsuture 7-0 and fibrin glue are used to hold the periosteum to the defect and to create a watertight seal.
For delivery of BMSCs via intra-articular injections, cultured cells are trypsinized and washed as mentioned earlier and resuspended in 0.5 to 1 mL of autologous serum in a sterile container. This, along with 2 mL of HA, is injected into the intra-articular space with a 19-gauge needle under local anesthesia.
Complications
Preliminary results from the use of MSC injections show that it is a safe procedure (in terms of serious adverse events during administration of MSC) and the rate of microbial contamination is low (1 in 218 processes during a 6-year span from 2006 to 2011).
Follow-up
Patients are reviewed on the 10th postoperative day for wound inspection and again at 3 weeks. The intra-articular injection of the cultured MSCs is given at this visit.
Patients are followed up for the first 6 months at 6 weekly intervals then at 1-year and 2-year intervals. The ICRS Cartilage Injury Evaluation Package36 is used for patients who underwent cell-based treatment for OCD. The package included questions from the International Knee Documentation Committee (IKDC) scores, the knee scale reported by Lysholm and Gillquist,37 and the Tegner and Lysholm Knee score.38 MRI is performed after 1 year to look for cartilage regeneration based on the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) scoring system.39 MRI was performed on a 1.5T Signa HDxt MRI scanner (GE Medical Systems, Milwaukee, WI) with a dedicated extremity surface coil (HD TR knee PA). The normal knee sequences used were axial T2, coronal proton density fat saturated, coronal T1, sagittal proton density, and sagittal T2 fat saturated. The cartilage sequences used were axial spoiled gradient recalled (SPGR), sagittal SPGR, axial fast imaging employing steady-state acquisition (FIESTA), and sagittal FIESTA. Each MRI scan consists of 19 slices for the normal knee protocol and 32 slices for the cartilage sequences. All patients were positioned consistently with the joint space in the middle of the coil and the knee extended in the coil.
Rehabilitation
The goal of rehabilitation is to facilitate recovery of the patient’s range of motion/mobility, walking, and cardiovascular capacity and strength; strict adherence to the protocol is encouraged to derive maximum benefits from the treatment.
The process begins on postoperative day 0 and includes passive range of motion and isometric muscle contractions. Regular continuous passive mobilization (CPM) is prescribed for 8 hours per day until 4 weeks postoperatively, starting at 30 degrees with gradual increments. This is made available during the inpatient stay and for rental when the patient is discharged. Patients unable to be reimbursed for CPM would undergo physiotherapy with 500 flexion and extension exercises per day. Patients are kept on non–weight bearing for 6 weeks, after which they are allowed active motion and partial weight bearing as comfortable, with gradual progression to full weight bearing status after 12 weeks. Thereafter, the rehabilitation protocol focuses on restoring muscular function through the use of stationary bicycles, elliptical trainers and treadmills, and closed-chain exercises.
The rehabilitation protocol is customized according to the patient’s age, previous activity level, and the type of procedure performed.
Discussion
A retrospective review led by Teo et al.33 looked at 23 patients between 12 and 21 years of age, with ICRS grade 3 or 4 OCD lesions involving the patella. These lesions were treated using cell-based therapy in conjunction with distal realignment or lateral release surgery from 2001 to 2008.
Twenty patients had ACI and three patients had cultured bone marrow stem cell implantation. The mean IKDC score, Tegner-Lysholm outcomes, and Lysholm-Gillquist scale improved from 45, 2.5, and 50, respectively, at surgery to 75, 4, and 70, respectively, at 24-month follow-up. However, not all patients returned to the activity level expected for their age. Additionally, two of the patients who had ACI were found to have asymptomatic periosteal hypertrophy noted on MRI scanning as early as 6 months follow-up. The periosteum appeared to have overgrown the defect likely as a result of cartilage hypertrophy and remodelling, and this complication has been well documented in the literature in patients treated with ACI.40,41
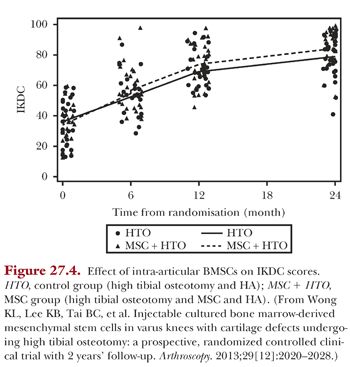
ACI have shown to be not superior to BMSCs in the treatment of cartilage defects but with increased complications such as donor site morbidity, periosteal pain, and periosteal hypertrophy.30 In our use of BMSCs, we are moving increasingly toward an intra-articular delivery instead of direct implantation via surgery, as we found no benefits with using an open technique of delivery.32 The usage of intra-articular injections, in addition, has the advantage of being minimally invasive and requires only a single operation. Intra-articular injections of BMSCs in HA have been compared with just HA in patients with varus knees and cartilage defects who underwent high tibial osteotomy and microfracture.42
Results at 2-year follow-up of 56 patients (28 cell recipient, 28 control) show that the cell-recipient group had significantly better scores (Figs. 27.4 to 27.6). The effect of treatment showed an added improvement of 7.65 (95% confidence interval [CI], 3.04 to 12.26; P < .001) for IKDC scores, 7.61 (95% CI, 1.44 to 13.79; P < .016) for Lysholm scores, and 0.64 (95% CI, 0.10 to 1.19; P < .021) for Tegner scores. MRI scans performed 1 year after surgical intervention showed significantly better MOCART scores for the cell-recipient group. The age-adjusted mean difference in MOCART score was 19.6 (95% CI, 10.5 to 28.6; P < .001).