PHYSIOLOGIC CHANGES OF PREGNANCY
Profound physiologic changes of the cardiovascular, respiratory, renal, and coagulation systems occur during pregnancy. It is important for the surgeon to understand these changes in order to understand normal from abnormal findings because they can affect laboratory interpretation, blood product replacement, and surgical approach.
Cardiovascular System
During pregnancy, maternal blood volume increases by 45% to 50% above nonpregnant volumes. Placental hormone production stimulates maternal erythropoiesis, increasing red cell mass by approximately 20%. Because plasma volume increases disproportionately to the increase in red blood cell mass, physiologic hemodilution occurs, manifested as a physiologic anemia. Pregnancy should be considered a hypervolemic state. A mild increase in maternal heart rate begins early in pregnancy and continues until term. In late pregnancy, maternal heart rate is increased by approximately 20% over antepartum values, often resulting in mild tachycardia.
Systemic vascular resistance decreases by 20%, but gradually increases near term. This results in a decrease in systolic and diastolic blood pressure during pregnancy, with a gradual recovery to nonpregnant values by term. As there is increased pressure in the venous system, there is decreased return from the lower extremities, resulting in dependent edema.
Respiratory System
In pregnancy, minute volume is increased, while functional residual volume is decreased. This is primarily the result of upward displacement of the diaphragm. It seems intuitive that lung volume would be decreased during pregnancy, but an increase in minute volume in association with an expansion of the anterior and posterior diameter of the chest results in increased tidal volume, thereby also increasing minute ventilation. These changes result in a compensated respiratory alkalosis. Normal Pco2 in pregnancy ranges from 28 to 35 mm Hg. Po2 is usually greater than or equal to 100 mm Hg. Oxygen consumption and basal metabolic rate are also increased during pregnancy by approximately 20%.
These physiologic changes result in less pulmonary reserve for the acutely ill pregnant patient; therefore, this reduces the time interval from respiratory distress to respiratory failure. Because of this, early recognition and intervention in patients with respiratory challenge is mandatory.
Gastrointestinal Tract
During pregnancy, there is a decrease in gastrointestinal motility. This is caused by mechanical changes in the abdomen with the enlarging uterus and smooth muscle relaxation induced by high production of progesterone in pregnancy. Gastric emptying may be delayed for up to 8 hours; therefore, pregnant women should be considered to have a functionally full stomach at all times. In addition, a decrease in large intestine motility may result in constipation severe enough to cause significant abdominal pain.
Coagulation Changes
Pregnancy is a hypercoagulable state. Fibrinogen is increased approximately 30% over baseline values. The hypercoagulable
state of pregnancy is associated with increased risk of deep venous thrombosis and pulmonary embolus. This is particularly compounded when bed rest or immobilization occurs during the gestational period.
Renal Changes
Pregnancy increases blood flow to the renal pelvis approximately 60% to 80%. This results in an increased glomerular filtration rate accompanied by frequent urination. Serum creatinine is approximately 40% less than in a nonpregnant state. Therefore, a creatinine of 1 mg/dL during gestation should be considered to be at the upper limits of normal.
Ureteral diameter increases in pregnancy secondary to compression and smooth muscle relaxation. Peristalsis is delayed, and reflux occurs freely from the bladder into the lower ureteral segment. This results in an increased incidence of pyelonephritis during pregnancy, making treatment of significant asymptomatic bacteriuria mandatory.
IMAGING TECHNIQUES
The most common imaging technique used during pregnancy is ultrasonography. Ultrasound is considered safe and primarily is used for fetal assessment. In patients with abdominal pain, an ultrasound should be considered the first-line diagnostic imaging test. During ultrasound, the presence of an intrauterine pregnancy should be documented and the size and other pertinent parameters of fetal well-being should be recorded. Evaluation of the cul-de-sac for fluid, the ureter for dilatation or stones, the gallbladder for the presence of gallstones, and the placenta for abnormalities should also be noted in the report.
Magnetic resonance imaging can be safely used during pregnancy. There are no data to suggest any increased fetal risk from this modality. Magnetic resonance imaging is now used to diagnose fetal abnormalities, especially abnormalities of the central nervous system. It may be particularly important for the diagnosis of appendicitis in pregnancy.
Although there are theoretical risks associated with ionizing radiation, fortunately, most diagnostic x-ray procedures are associated with minimal or no risk to the fetus. While unnecessary, multiple, or recurrent x-ray exposure should be avoided, existing evidence suggests that there is no increased risk of fetal congenital malformations, growth restriction, or abortion from x-ray procedures that expose the fetus to doses of 5 rads or less. The American Congress of Obstetricians and Gynecologists has published guidelines regarding diagnostic imaging during pregnancy. Women should be reassured that concern about radiation exposure should not prevent medically indicated diagnostic procedures. It cannot be stressed enough that maternal well-being is of the utmost importance, and appropriate diagnostic procedures should be obtained to facilitate a rapid diagnosis.
POSTPARTUM HEMORRHAGE
Postpartum hemorrhage is poorly defined by estimation of blood loss; therefore, it is difficult to accurately determine actual or percentage of blood loss at the time of delivery. Additionally, blood volume expansion is variable during pregnancy and can be affected by several factors including hypertension, renal disease, maternal body mass index, and the presence of multifetal gestations. The potential effects of blood loss largely depend on the degree of blood volume expansion. For example, blood loss of 1,000 mL during cesarean section is generally well tolerated by healthy pregnant women. A blood loss of 500 to 750 mL, however, may not be tolerated in a woman with minimal volume expansion or one who is hemoconcentrated secondary to severe preeclampsia or eclampsia. The American Congress of Obstetricians and Gynecologists has noted that a number of definitions have been used to define hemorrhage, including a blood loss greater than 500 mL with a vaginal delivery or greater than 1,000 mL during a cesarean section or a drop in hematocrit of 10% regardless of the amount of documented blood loss.
Gilstrap and Ramin have defined clinically significant hemorrhage as that amount of bleeding “that produces signs and symptoms of hemodynamic instability or that is likely to produce such if left unabated.”
Incidence and Etiology
Although the exact incidence of hemorrhage associated with pregnancy is unknown, it remains one of the leading causes of maternal mortality in this country. Kaunitz and associates reported that 13% of more than 2,000 maternal deaths were secondary to hemorrhage, and one third of these occurred postpartum. Rochat and colleagues reported a similar incidence of 11% of maternal deaths resulting from hemorrhage.
In a randomized trial conducted in the United States, birth weight, labor induction and augmentation, chorioamnionitis, use of magnesium sulfate, and a maternal history of previous obstetrical hemorrhage were associated with increased risk of postpartum hemorrhage. A large population-based study supported these findings, with significant risk factors identified using multivariate analysis. Risk factors associated with an increased risk of postpartum hemorrhage were retained placenta (odds ratio [OR] 3.5; 95% confidence interval [CI]: 2.1 to 5.8), failure to progress during the second stage of labor (OR 3.4; 95% CI: 2.4 to 4.7), placenta accreta (OR 3.3; 95% CI: 1.7 to 6.4), vaginal or perineal lacerations (OR 2.4; 95% CI: 2.0 to 2.8), instrumental delivery (OR 2.3; 95% CI: 1.6 to 3.4), large-for-gestational-age newborn (OR 1.9; 95% CI: 1.6 to 2.4), hypertensive disorders (OR 1.7; 95% CI: 1.2 to 2.1), induction of labor (OR 1.4; 95% CI: 1.1 to 1.7), and augmentation of labor with oxytocin (OR 1.4; 95% CI: 1.2 to 1.7).
Diagnosis and Medical Management
The most important aspects in the management of postpartum hemorrhage are prompt recognition and treatment of the condition. Recognition of external bleeding is straightforward and almost always can be controlled with medical or minor surgical means. Tachycardia, decreased urine output, and, finally, hypotension without obvious external blood loss are important signs of potential internal bleeding, and many such instances will require surgical intervention to arrest the hemorrhage. If the etiology of the hemorrhage is not determined quickly, coagulopathy may complicate the clinical presentation, thereby making diagnosis and treatment more difficult.
Uterine atony is readily recognizable by palpation of the uterus. If atony it not present, the cervix and vagina should be carefully inspected for lacerations. The placenta also should be inspected for missing fragments, and careful manual palpation of the uterine cavity should be performed. If the source of bleeding still is not obvious or if bleeding is seen around venipuncture or catheter sites, then the patient should be evaluated for coagulopathy. A thrombin clot (clot retraction test) tube will reveal gross disruption in coagulation within minutes. Useful laboratory tests include prothrombin time, partial thromboplastin time, platelet count, fibrinogen, and fibrin degradation product levels. The clinician should note that d-dimer levels may be abnormal during pregnancy even in patients without coagulopathy. Before surgical intervention, an ultrasound
examination of the uterine cavity may prove useful for the identification of an accessory placental lobe or fragment.
Medical management of postpartum hemorrhage consists of volume replacement and uterotonics, including intravenous oxytocin and parenteral methylergonovine and prostaglandins. Volume can be maintained with crystalloid and blood or blood products. Invasive monitoring, such as with a pulmonary artery catheter, generally is not necessary and may be dangerous in the presence of a coagulopathy. Its use should be reserved for those patients who do not respond to usual and expected therapy. As a general rule, monitoring of urine output, vital signs, and oxygen saturation will be sufficient. Volume replacement generally is adequate when the blood pressure is maintained at 90 to 100 mm Hg systolic, pulse rate is less than 100 beats per minute, and urine output is at least 25 to 30 mL per hour. When a patient has required transfusion of packed red blood cells, it is important to transfuse coagulation factors to replace those lost in the hemorrhage. Calcium also should be replaced in these patients because of risk of complications such as hypotension related to hypocalcemia in patients receiving massive transfusions. Fluid overload generally can be detected with a stethoscope and an oxygen monitor in conjunction with clinical signs and symptoms. Diuretics should only be used to remove excess fluid if the patient becomes hypoxic related to volume overload. A medical management protocol for postpartum hemorrhage is summarized in
Table 35A.1.
Surgical Management
Lower genital tract lacerations usually are best managed by suturing. The rare case of uterine rupture also is managed surgically. Other techniques to control hemorrhage include uterine and utero-ovarian artery ligation, hysterectomy, or uterine or hypogastric artery embolization.
Uterine packing, which until recently had been abandoned by most clinicians, may allow adequate time for blood and fluid replacement before surgical intervention. Maier described the use of a packing device called a Torpin packer. The device uses a plunger to place several yards of 4-inch-wide gauze into the uterine cavity and has been used successfully to control postpartum hemorrhage. Uterine packing allows time for volume replacement and slows bleeding enough to allow for surgical techniques short of hysterectomy. In many cases, it may stop the bleeding so that no further treatment is necessary. Other methods of uterine tamponade reported in the literature have included the use of a Foley catheter with a 30- to 50-mL balloon or a Sengstaken-Blakemore tube with the esophageal balloon inflated with 50 mL of normal saline and, more recently, the use of the Bakery balloon. The choice of a specific surgical technique to control bleeding depends on several factors, including the degree of hemorrhage, the hemodynamic status of the patient, parity, and the desire for future childbearing. An undoubtedly important factor in achieving a favorable outcome in control of postpartum hemorrhage is the experience of the surgeon.
Arterial Embolization
Angiographic arterial embolization has been described for the successful control of obstetric and gynecologic bleeding. Small metal coils, Gelfoam, polyvinyl alcohol dehydrated particles, and other substances have been used for such embolizations. Pelage and associates, in two separate reports, describe use of arterial embolization in patients with primary or secondary postpartum hemorrhage, defining primary postpartum hemorrhage as that which occurs within 24 hours after delivery. Twenty-seven women were identified, including two who had already undergone hysterectomy in an unsuccessful attempt to control the hemorrhage. Following transcatheter embolization, immediate decrease or cessation of bleeding occurred in all patients. Two patients required repeat embolization the next day with no further complications. Fourteen women were diagnosed with secondary postpartum hemorrhage after the first 24 hours following delivery. All of these patients had complete resolution of bleeding with embolization with no further complications. Arterial embolization can be performed quickly and safely; therefore, it should be strongly considered in patients with postpartum hemorrhage who are stable enough to be managed in the radiology suite. There have been reports of successful pregnancies following embolization, which makes it an especially attractive alternative to hysterectomy in the patient who desires preservation of fertility.
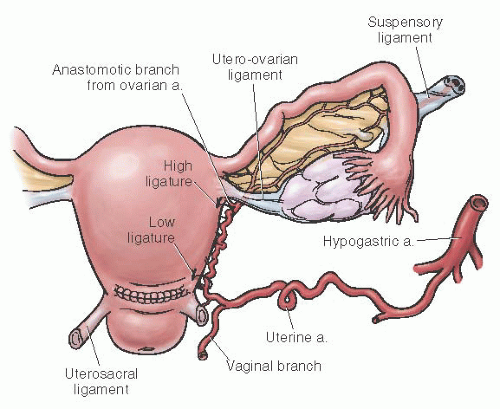
Uterine Artery Ligation
Uterine artery ligation is a relatively safe procedure that can be performed by most obstetricians. It also allows for future childbearing. The technique consists of ligating the uterine artery and vein at the lower uterine segment 2 to 3 cm below the level of the transverse uterine incision. An
absorbable ligature is placed 2 to 3 cm medial to the uterine vessels through the myometrium (to obliterate any intramyometrial ascending branches) and then lateral to the vessels through the broad ligament. It is imperative that the bladder be advanced before placement of the suture to prevent bladder injury. Because of collateral flow from the ovarian artery, some recommend that a second ligature be placed at the junction of the utero-ovarian ligament and uterus. The technique of uterine artery ligation is shown in
Figures 35A.1 and
35A.2.
In a review of 90 women who underwent uterine artery ligation (30 were for uterine atony), O’Leary reported that only six (7%) procedures resulted in failure. There were no major complications from the procedure itself. In a follow-up review of 265 women who underwent uterine artery ligation, O’Leary reported a greater-than-95% success rate.
This technique is most useful (and successful) when hemorrhage is of a moderate degree and originates from the lower uterine segment. Such an example is bleeding from a low placental implantation site. Uterine artery ligation also can prove beneficial for lower segment extensions or lacerations, in addition to slowing bleeding for a uterine artery laceration. Philippe and associates reported a vaginal approach to ligation of the uterine arteries in two patients after vaginal delivery, but a larger case series would have to be performed to determine the feasibility of this approach.
B-Lynch Suture
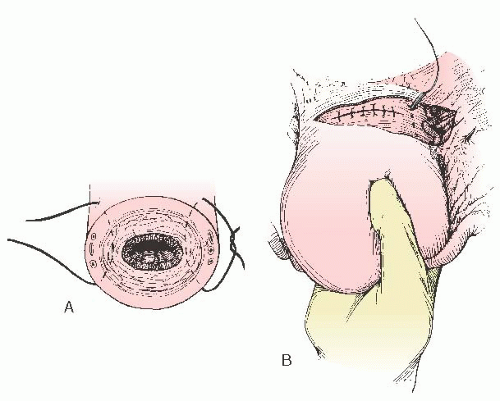
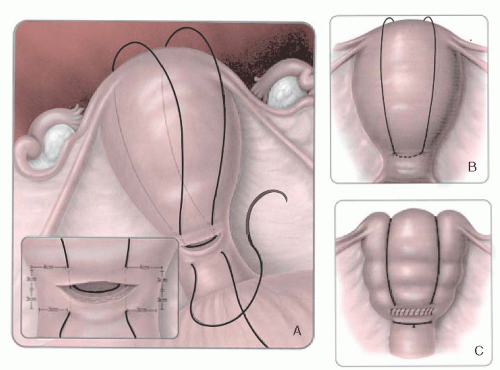
B-Lynch and colleagues also described five cases in which hemorrhage was controlled by placing an absorbable suture vertically from 3 cm below the uterine incision to 3 cm above the uterine incision on the right side of the uterus (
Fig. 35A.3). The stitch is then taken vertically over the fundus and placed horizontally in the posterior uterus at the same level as the anterior suture. The suture is threaded over the left side of the uterus to place another stitch on the left from 3 cm above the uterine incision to 3 cm below the uterine incision. The long suture is tied, compressing the fundus. A large suture, such as no. 1 Prolene on a large needle, is used. The uterine incision is closed in the usual fashion. There are several case series in the literature supporting the efficacy of the B-Lynch stitch for the treatment of uterine atony. In all series, the suture has been reported to be effective. It has been suggested that the B-Lynch be considered in all cases of severe postpartum hemorrhage before resorting to hysterectomy.
Hypogastric Artery Ligation
The major blood supply to the uterus and pelvis comes from the internal iliac artery, often called the hypogastric artery. Bilateral ligation of this artery can effectively control significant bleeding and thus prevent the need for hysterectomy and permanent sterilization. Burchell has aptly described the physiology of internal iliac artery ligation. It appears that ligation of this artery controls bleeding by converting an arterial system into a venous system, which decreases the pulse pressure by as much as 85%. This allows pressure and packing to produce clotting. Hypogastric artery ligation probably interferes little, if at all, with subsequent pregnancies. Mengert and colleagues reported successful pregnancies in five women who had undergone internal iliac artery ligation. This technique also may prove useful for controlling bleeding in patients with large hematomas of the broad ligament or for a lacerated artery that has retracted into the broad ligament. Such vessels or active bleeding sites often are difficult to identify. If the bleeding is from the hypogastric vein, ligation of the hypogastric artery will decrease venous pressure which makes the bleeding easier to control.
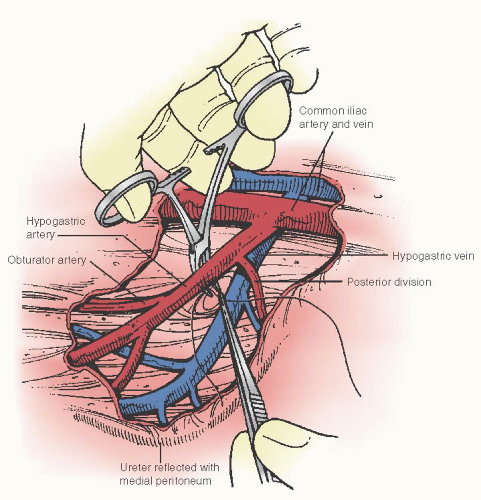
The technique of hypogastric artery ligation is illustrated in
Figure 35A.4. The peritoneum overlying the external iliac artery is divided directly above the artery between the infundibulopelvic ligament and the round ligament of the uterus. The internal iliac (hypogastric) artery is identified as it arises and runs from the common iliac artery posteriorly into the pelvis just beneath the infundibulopelvic ligament. The ligation should be performed about 2 cm distal to the bifurcation to avoid disrupting the posterior division of the hypogastric, which can lead to ischemia and necrosis of the skin and subcutaneous tissue of the gluteus. A right angle clamp is gently passed under the artery in the lateral to medial direction with blunt dissection. Great care must
be taken not to perforate the internal iliac vein, and the clamp is passed lateral to medial to avoid injury to the vein by the tip of the clamp. Two nonabsorbable sutures of 2-0 silk should be used for ligation. It is important that hypogastric artery ligation be performed bilaterally to adequately decrease systolic pressure to the uterus. Clark and associates reported on the successful control of bleeding in 8 (42%) of 19 women who underwent hypogastric artery ligation. In a review of hypogastric artery ligation from three series, Clark reported that this procedure prevented hysterectomy in about half of the patients associated with uterine atony and placenta accreta. Interestingly, in this series, the success of hypogastric artery ligation did not appear to be related directly to the conditions for which it was performed. It must be noted, however, that the number of patients in each category is small.
Although this procedure is successful in about 50% of patients and does not interfere with subsequent fertility, it is not technically easy to perform and requires special skill and experience. Many obstetricians have little, if any, experience with this procedure, especially in the presence of a surgical emergency. Moreover, potential complications of hypogastric artery ligation include laceration of the iliac vein, ligation of the external iliac artery, ureteral injury, and death.
Hysterectomy
Because of the lack of experience and skill with the technique of hypogastric artery ligation, many clinicians prefer to do a hysterectomy to control postpartum hemorrhage. Peripartum hysterectomy is extensively discussed later in this chapter. Hysterectomy usually is the safest procedure and also the quickest that can be performed for refractory bleeding. For example, Clark and associates reported that patients undergoing hypogastric artery ligation who subsequently required hysterectomy had an increased incidence of cardiac arrest secondary to blood loss. The increased morbidity associated with hypogastric artery ligation followed by hysterectomy may be secondary to a delay resulting from attempted conservative management short of hysterectomy. Hypogastric artery ligation was attempted before hysterectomy 64% of the time in nulliparous women, compared with 10% of the time for multiparous patients. Lack of experience with hypogastric artery ligation adds to the overall time required to attempt the procedure and overall blood loss.
In a review of 70 women who underwent emergency hysterectomy for postpartum hemorrhage, Clark and colleagues reported that almost all required blood transfusion, and 50% had postoperative febrile morbidity. The most common indication for hysterectomy in this series was uterine atony, followed by placenta accreta. Of the 70 procedures, 60 were performed after cesarean delivery. Mean operating time was 3.1 hours, and mean blood loss was 3,575 mL.
ABNORMAL PLACENTATION
Placenta accreta occurs when the placenta attaches abnormally to the underlying uterine wall. It is believed to occur due to an abnormal attachment of the chorionic villi to the myometrium due to an absence of decidua basalis and incomplete
development of the fibrinoid layer. Placenta increta occurs when the placenta extends in to the myometrium, and placenta percreta occurs when the placenta invades through the myometrium and serosa with possible involvement of adjacent organs. Because of the abnormal attachment to the myometrium, there is a risk of life-threatening hemorrhage often requiring massive transfusion of blood products and hysterectomy. The maternal mortality rate was reported in 1996 by O’Brien and his group to be as high as 6% to 7%.
Flood and his group reported in 2009 that the incidence of placenta accreta has increased from approximately 0.8 per 1,000 deliveries in the 1980s to 3 per 1,000 in the past decade. The rising incidence of placenta accreta parallels the rising cesarean section rate. Important risk factors include prior history of placenta previa, previous cesarean delivery, increasing parity and maternal age, and prior uterine surgery. Antepartum diagnosis of placenta accreta is best accomplished by ultrasound, with a reported sensitivity of 77%, specificity of 71%, and a positive predictive value of 65%. Sonographic findings that may be associated with accreta include loss of normal hypoechoic retroplacental myometrial zone, thinning of the uterine serosa-bladder interface, and increased vascularity at the interface of the uterus and bladder. Magnetic resonance imaging may be helpful if there is suspicion that the placenta has invaded the parametrium or surrounding organs.
LATE POSTPARTUM HEMORRHAGE
Late postpartum hemorrhage is defined as occurring more than 24 hours after delivery. The etiology of such bleeding includes placental site subinvolution, infection, coagulopathy, and retained products of conception. Initial therapy for this complication is the same as for early hemorrhage. If infection is present, antibiotics should be used. Endometrial curettage may be necessary for retained placental fragments. Angiographic embolization may prove especially useful in the case of late postpartum hemorrhage. Uterine artery ligation, hypogastric artery ligation, and hysterectomy are rarely required for control of late postpartum hemorrhage.
PERIPARTUM HYSTERECTOMY
Horatio Storer performed the first cesarean hysterectomy in 1869. Initially, the procedure was performed only for emergency situations, but in the early 20th century, it became an accepted means of sterilization. In the modern obstetrical age, elective cesarean hysterectomy is rarely performed, except in cases of cervical neoplasia.
Peripartum hysterectomy can be performed in conjunction with a cesarean delivery (e.g., cesarean hysterectomy) or after a vaginal delivery for complications such as postpartum hemorrhage. In a recent review of a nationwide sample of deliveries from 1998 to 2003, Whiteman and colleagues estimated that the rate of peripartum hysterectomy in the United States is 0.77 per 1,000 deliveries.
Although there is little controversy regarding peripartum hysterectomy for emergency conditions, there is significant debate in modern obstetrics regarding an elective hysterectomy performed at the time of cesarean delivery. There has been legitimate concern about increased morbidity related to peripartum hysterectomy—including damage to the ureters, bladder, and rectum—and an increased rate of reoperation. However, Plauche has pointed out that morbidity often is associated with the conditions leading to the hysterectomy and not necessarily the procedure itself. Lower morbidities have been reported for elective cesarean hysterectomies when compared with emergency hysterectomies. However, there is inherent bias in these retrospective reviews. Emergent surgery for lifesaving maternal indications would be expected to have higher morbidities, such as blood loss and injury to surrounding structures. In a retrospective study, Castaneda and colleagues observed that over the years the indications for peripartum hysterectomy have changed from predominately elective to almost exclusively emergent indications. In their series, the average blood loss was 3,009 mL in emergent cases and 1,262 mL in nonemergent cases. They concluded that, at the present time, peripartum hysterectomy is almost always emergent in nature and associated with a significant blood loss. As might be expected, there are no randomized prospective studies of elective cesarean hysterectomy, and it is unlikely that such a study could ever be done given the ethical dilemma involved.
Emergency Peripartum Hysterectomy
Obstetric hemorrhage secondary to a variety of etiologies is a common indication reported for peripartum emergency hysterectomy. The three most common reasons are uterine rupture, abnormal placentation, and uterine atony. Although the exact incidence of emergency peripartum hysterectomy is not known, several authors have reported widely varying rates of 0.004 to 1.5 per 1,000 deliveries.
Clark and associates reviewed 70 cases of emergency hysterectomy for obstetric hemorrhage and found that 60 (86%) of these procedures were performed after cesarean delivery and 10 (14%) were performed after vaginal delivery. Uterine atony and placenta accreta accounted for almost three fourths of the cases. Other indications were uterine rupture, extension of the uterine incision, and fibroids precluding closure of the uterine incision.
It is clear from the literature that
abnormal adherent placentation or placenta accreta (with or without hemorrhage) is emerging as the most common condition leading to an emergency hysterectomy. In three studies from 1993 to the present, 156 (56%) of the 279 emergent peripartum hysterectomies were performed for placenta accreta. These studies are outlined in
Table 35A.2.
The increase in placenta accreta is related to the high rate of cesarean deliveries, which has risen in the United States from 5% in the early 1960s to 30% at the present time. A recent study of more than 60,000 deliveries at the University of Chicago by Wu et al. found that the rate of placenta accreta had increased to 3 per 1,000 deliveries in 2003. They observed that this was directly associated with the increase in cesarean section rate. Clarke and colleagues found that in the presence of a placenta previa, the risk of having placenta accreta increased from 24% in women with one prior cesarean delivery to 67% in women with 3 or more prior cesareans.