EMBRYOLOGY
The reproductive system is derived from mesoderm. The primordium of the urogenital ridge is divided into two segments. One is the nephrogenic ridge, that is, metanephros derivatives, the renal system; the other is the gonadal ridge for development of the reproductive tract. Gonads are a reflection of three origins: mesothelium, mesenchyme, and primordial germ cells. The paramesonephros gives rise to the fallopian tubes and the uterus. Two gonadal ridges arise early in gestation (4 to 5 weeks) in the
developing embryo as thickening on the medial aspect of the coelomic cavity adjacent to the mesonephros. These gonadal outgrowths are composed of coelomic epithelium and underlying mesenchyme projecting into the future peritoneal cavity. The epithelial and mesenchymal cells of the gonadal primordia are of mesodermal origin (large, spherical ovoid germ cells that originate extragonadally in the wall of the yolk sac and migrate to the developing gonads). Until the 6th week of gestation, the gonads of the two sexes remain morphologically indistinguishable. The presumptive ovaries remain undifferentiated until the onset of meiosis at the end of the first trimester. The ovarian cortex is a single germinal epithelium. The tunica albuginea lies beneath the cortex and is composed of connective tissue. The stroma is composed of fibroblasts, smooth muscle, endothelium, and interstitial cells, including undifferentiated theca cells and corpora albicans.
Sexual differentiation requires initiation by various genes, along with a single gene determinant on the Y chromosome (testis-determining factor), which is necessary for testicular differentiation. In XX individuals (in the absence of a Y chromosome), the bipotential gonad develops into an ovary.
The mechanisms responsible for gonadal sex differentiation are largely unknown. Investigators have theorized the presence of a testis-determining factor (H-Y cell-surface antigen on the short arm of the Y chromosome) that is elaborated by a specific gene. Meiosis-inducing and meiosis-preventing substances, both of which are produced by cells derived from mesonephric structures adjacent to the gonad, are the agents of regulation of ovarian and testicular germ-cell differentiation. The balance between these two substances varies between the two sexes and at different stages of development. The meiosisinducing substance predominates in the fetal ovary. Maternal ovarian hormone production is not required for differentiation of the germ cells or, apparently, for later development of the fetal reproductive tract. Various ultrastructural studies have shown no specific changes in fetal granulosa cells that can be definitely associated with steroid hormone secretion such as is identified in the fetal Leydig cells. Thecal cells play an essential role in steroid synthesis in the adult ovary, but they do not appear until later in gestation and even then retain a relatively undifferentiated appearance. Fetal pituitary gonadotropin production begins as early as 10 weeks gestation and reaches peak levels at midgestation. Gonadotropins have a major influence on follicular development in the adult ovary, but evidence for a similar function in the fetus is lacking.
GENE EXPRESSION
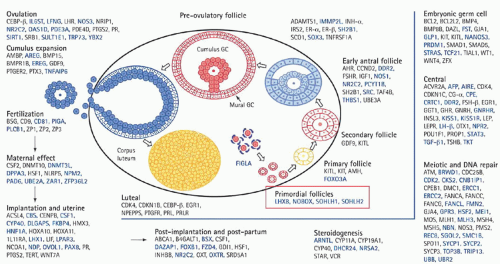
Understanding of the genetics of the ovary continues to evolve. With the advent of polymerase chain reaction (PCR), real-time PCR, fluorescence in situ hybridization, single nucleotide polymorphism, and a host of other genetic advances, understanding of ovarian function has reached a new level (
Fig. 28.1).
Table 28.1 provides information regarding genes and associated phenotype.
Genes often are associated with specific clinical problems, for example, Kallmann syndrome, a genetic condition that results in the failure of pubertal development. Hypogonadism associated with a total lack of sense of smell (anosmia) or a heavily reduced sense of smell (hyposmia) characterizes the syndrome. Premature ovarian insufficiency (POI) is characterized by hypergonadotropic hypogonadism, which equates with the loss of ovarian function before age 40. The problem of POI overall is associated with a host of gene defects, all of which set the stage for genetic testing in patients in the reproductive age group with amenorrhea in association with hypergonadotropic state. Genes are also involved with cumulus expansion (GDF9 and BMP15) and endometriosis, and most recently serve as predictors of oocyte quality and successful embryo implantation and development. Specific follicular cell receptors bind growth factors, which are locally synthesized with the ultimate effect of intracellular signaling and protein kinase activation. This activity affects transcription of targeted genes. Gene expression is involved in follicle development, ovulation,
and corpus luteum and corpus albicans formation. Transcription factors include protooncogenes, c-Myc, and CCAAT/enhancer binding protein.
FEMALE FETAL DEVELOPMENT
The ovarian surface cortex, during the early prefollicular stage, is characterized by germ cells and granulosa cells organized in cords and sheets, but the cortex lacks specific conformation. The final distinctive change to occur in the fetal ovary is the onset of meiosis at the 11th or 12th week of gestation. Meiosis is preceded by differentiation of primitive germ cells into actively dividing mitotic cells called oogonia. The mitotic divisions of the oogonia are associated with complete separation at telophase, leaving the daughter cells connected by intracellular bridges. After a series of mitotic divisions, there is progressive entry of cells into meiosis, beginning in the innermost cortex and gradually extending to the periphery. These cells passing through the various stages of the first meiotic prophase are then designated oocytes. By late gestation, all surviving oocytes have advanced to the diplotene stage. Further differentiation of the oocytes is arrested at this stage and does not resume until ovulation begins at menarche, approximately 12 years later.
Follicular formation begins at 18 to 20 weeks gestation and continues throughout the remaining weeks of fetal development. All the surviving oocytes are surrounded by adjacent granulosa cells; oocyte and follicular growth are well established by the late fetal and early neonatal period. The constant degeneration and loss of oocytes before their incorporation into the follicles reduces their numbers to only 1 to 2 million (follicles) in the newborn ovary.
ANATOMY
The dimensions of the adult ovary vary from individual to individual but average 3 to 5 cm in length, 2 to 3 cm in width, and 1 to 2 cm in diameter, with a weight of 3 to 8 g. The ovarian capsule is smooth in childhood, but its surface becomes pitted from follicular maturation and atresia.
The size, shape, and position of the ovary in the pelvis are somewhat variable, and both the consistency and the follicular changes taking place within the ovary vary with stage of the menstrual cycle. The ovary typically is anchored to the sidewall of the pelvis in the shallow peritoneal fossa of Waldeyer formed between the angles of proximity of the ovary to the ureter. This knowledge is important before dissecting the ovary off the pelvic sidewall.
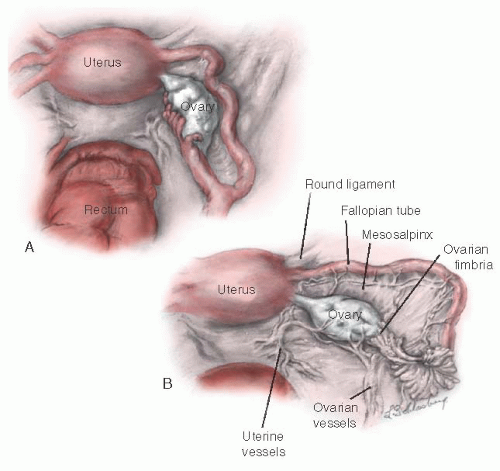
The ovary is connected to the uterus by the utero-ovarian ligament, to the posterior aspect of the broad ligament by the mesovarium ligament, and to the lateral pelvic sidewall by the infundibulopelvic ligament (
Fig. 28.2). The
mesovarium ligament attaches to the mesentery of the ovary. The other two ligaments are attached at the hilum of the ovary.
The ovary migrates downward from high in the abdomen during embryonic life. The infundibulum of the fallopian tube extends onto the ovary and is attached to it at its most distal pole by the fimbria ovarica. The relation of the ovary to the fimbria ovarica and to the utero-ovarian ligament is crucial, and they should be carefully maintained during ovarian reconstruction.
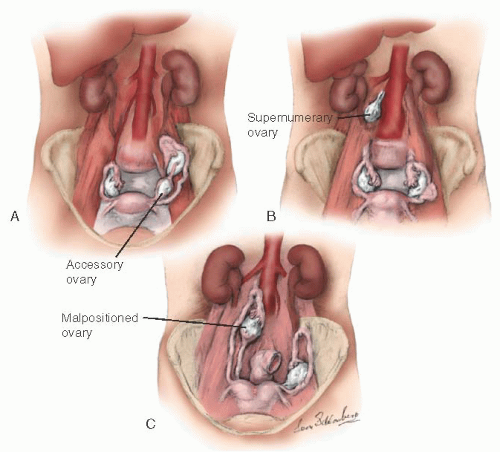
During embryogenesis, the ovary may assume an unusual appearance (i.e., it may be septate) or assume an unusual position (
Fig. 28.3). An accessory ovary (
Fig. 28.3A) usually is close to or is connected to a normally placed ovary. An accessory ovary also may be attached to the broad, utero-ovarian, or infundibulopelvic ligaments. Unlike the accessory ovary, a supernumerary ovary (
Fig. 28.3B) must have an independent embryologic origin. It may develop from a primordium such as arrested migrating gonadocytes. A supernumerary ovary consists of typical ovarian tissue but has no direct or ligamentous connection with a normally placed ovary. A supernumerary ovary is thus a true third ovary that has independent function and is located at a point that is distant to a normally placed ovary. Ovarian malposition (
Fig. 28.3C) also may occur when the ovary fails to descend into the pelvis to assume its normal location. In ovarian malposition, the ovary is attached as it should be to the uterus by the utero-ovarian ligament and to the fallopian tube by the fimbria ovarica, but it may lie adjacent to the liver or spleen. The ovary is elongated and may measure up to 15 cm in length. The fallopian tube attaching to such a malpositioned ovary may be 20 to 26 cm in length, almost twice its normal length.
The normal ovary has a surface covering composed of a single layer of flattened, germinal epithelial cells. This layer is contiguous at the ovarian hilum, with the peritoneal epithelium of the posterior leaf of the broad ligament. Beneath the germinal epithelium is a second layer of condensed ovarian stroma that forms a fibrous capsule, the tunica albuginea. The area through which the vessels and nerves enter and exit is the hilum of the ovary. Immediately around the hilum and extending into the substance of the ovary is an area known as the medulla, which is covered by the cortex. The medulla is composed of fibrous tissue unlike the condensed stroma of the ovarian cortex. The medulla contains no follicles; it has only blood vessels and the remnants of the tubular structure that would have developed into a testis (i.e., the rete ovarii) had the fetus been male.
The ovarian artery arises from the renal arteries. The artery descends from the aorta and crosses the ureter obliquely to enter the infundibulopelvic ligament on its course to the ovary. When it reaches the broad ligament, the ovarian artery branches to supply the fallopian tube and ovary before it finally anastomoses directly with the uterine artery to form a continuous arcade in the broad ligament. The ovarian veins are situated mainly in the mesosalpinx, where they give rise to the pampiniform plexus. At the outer end of the broad ligament, this plexus coalesces to form a single, large ovarian vein. The ovarian vein runs adjacent to ovarian artery to terminate in the inferior vena cava on the right and the renal vein on the left.
The lymphatic vessels of the ovary drain in three directions. The main group accompanies the ovarian vessels in the infundibulopelvic ligament and eventually reaches the periaortic nodes in the vicinity of the kidney. Other lymphatic channels communicate with channels of the opposite ovary by crossing the fundus of the uterus through the ovarian ligament. Some channels drain through the ovarian and round ligaments into the superficial inguinal lymph nodes in the groin. The ovary is supplied by both motor and sensory parasympathetic and sympathetic nerves,
which accompany the ovarian vessels from the abdomen as they pass into the infundibulopelvic ligament to reach the hilum of the ovary. The segmented nerves supply the ovary from T10 and T11.
ADNEXAL MASS
The uterine adnexa (gynecologic origin) consist of the ovaries, the fallopian tubes, and the uterine ligaments. Although adnexal pathology often involves one of these structures, contiguous tissue of nongynecologic origin also may be involved. Adjunctive diagnostic techniques such as sonography, magnetic resonance imaging (MRI), and computed tomography (CT) may help delineate the nature of adnexal enlargement. Pelvic ultrasonography, especially three dimensional, is an accurate means of determining the location, size, extent, and consistency of pelvic masses and is also useful for detecting obstructive uropathy, ascites, and metastasis. Other more specialized diagnostic procedures also may be necessary for the evaluation of an adnexal mass (
Table 28.1).
Computed tomography scanning has been particularly useful in gynecologic oncology because it helps define the extent of paracervical and parametrial involvement and allows a reasonable determination of the resectability of malignant neoplasms. Magnetic resonance imaging has surpassed CT in the precision of measurement of adnexal masses. Magnetic resonance imaging also allows a clear definition of the relationship of adjacent organs.
In a study conducted by Timmerman and coworkers, assessment was made of the use of both ultrasound and circulating levels of CA-125 antigen. Multivariate logistic regression analysis algorithms were used to distinguish benign adnexal masses from a malignant process. Transvaginal ultrasonography with color Doppler imaging was recorded in the 191 patients evaluated, aged 18 to 93 years. Of interest, 26.7% of the cohort of patients studied had malignant tumors. The authors believed that regression analysis could be used to accurately discriminate malignant from benign adnexal masses preoperatively.
An intriguing aspect of ultrasound assessment is the prediction of malignancy in adnexal masses using an artificial neural network. Taylor and colleagues reported generating a neural network algorithm that enabled computing of a probability of malignancy score for preoperative discrimination between malignant and benign adnexal masses. A retrospective analysis that included training in artificial neural network assessing transvaginal B-mode ultrasonography and color Doppler imaging was determined. The variables that were put into the artificial neural network included age, menopausal status, maximum diameter of the neoplasm, tumor volume, and papillary projections. The results identified four primary variables that were most effective in distinguishing benign versus malignant processes. These variables included age, time-averaged maximum velocity, papillary projection score, and maximum tumor diameter. The authors concluded that artificial neural networks are a useful clinical parameter to distinguish benign from malignant ovarian masses.
Surgical intervention ultimately may be necessary to determine the nature of the adnexal mass. Minimally invasive surgery is useful to exclude benign ovarian or nonovarian neoplasms. Indications for visualization and as indicated to obtain a tissue diagnosis of an adnexal mass with laparoscopy or exploratory laparotomy include the following:
Ovarian mass greater than 6 cm in diameter
Adnexal mass greater than 10 cm in diameter
Any solid mass first developing after menopause
Failure to discover the nature of the mass (e.g., leiomyoma) with radiologic or sonographic imaging techniques
One of the major goals of evaluation of the adnexal mass is to rule out malignancy. There is an age-dependent risk for a malignant adnexal mass. The incidence of malignant neoplasm increases significantly after age 50 years. Increased size of the adnexal mass is associated with an increased risk of malignancy.
Granberg and colleagues found that less than 1% of masses smaller than 5 cm were malignant, less than 11% of masses 5 to 10 cm were malignant, and 72% of masses larger than 10 cm were malignant. Sassone and associates, in an evaluation of women of all ages (mean age 41 years) by transvaginal sonography, found that 3% of masses smaller than 5 cm and 7% of masses 5 to 10 cm were malignant; the incidence of malignancy for masses larger than 10 cm was 13%.
Endometriosis is a common cause of an adnexal mass. An endometrial cyst of the ovary may develop into an endometrioma. Leakage of blood from the cyst may cause peritoneal irritation, pelvic adhesions, and pelvic organ fixation.
Tubo-ovarian inflammatory complex usually is the result of incompletely treated or unresolved subacute, chronic pelvic inflammatory disease (PID) in the walled-off area surrounding the pelvic structure.
Uterine leiomyomata cause nodularity and consequent irregular conformation of the uterus. The uterus may become enlarged and may present as an abdominal mass. The inability to distinguish a leiomyoma from an ovarian tumor on pelvic examination is an indication for further diagnostic evaluation.
Adnexal enlargement may be the result of carcinoma of the rectum, appendix, or bladder. Patients present with a variety of symptoms according to the organ involved. A complete and thorough evaluation is necessary to delineate the etiology of a neoplasm.
An adnexal mass may be noted in cases of acute abdomen. The differential diagnosis should include adnexal torsion, ruptured hemorrhagic cyst, degenerating leiomyomata, ectopic pregnancy, unruptured tubo-ovarian abscess, acute appendicitis with or without abscess formation, and diverticular disease of the sigmoid colon. A careful history, pelvic examination, and appropriate imaging studies often allow a prompt diagnosis.
Although every adnexal mass requires individual evaluation and management, it is possible to make a number of useful general recommendations. Expectant management is justified only when an asymptomatic physiologic cyst is suspected. Most cysts greater than 6 cm in diameter require a thorough evaluation. Imaging techniques are invaluable for characterizing the nature of the adnexal enlargement, but these procedures do not replace a careful medical history and thorough physical and pelvic examination.
ADNEXAL MASS DURING PREGNANCY
The incidence of adnexal mass in pregnancy requiring surgical intervention has been reported to occur at 1 in 81 to 2,500 pregnancies. When an adnexal mass is noted incidentally on ultrasound during pregnancy, the majority of small, simple cysts do not pose a risk to the pregnancy. Furthermore, most large or sonographically complex masses spontaneously resolve, as reported by Bernhard and colleagues; this study evaluated 18,391 ultrasound studies done in an obstetric population for which 432 women were identified with an adnexal mass. The incidence of adnexal masses was 2.3% in the pregnant population evaluated. In addition, the rate of torsion of the adnexal mass was 1%, and the rate of malignancy was also reported as 1%.
The majority of patients in one other study involving 320 pregnant patients with an adnexal mass were noted to have a simple cyst less than 5 cm, specifically 76%; the cysts were
more commonly asymptomatic. The remaining patients were noted to have complex cycsts greater than 5 cm; of interest, of the complex cysts, 69% resolved spontaneously. The concern always remains regarding the potential for malignancy. Masses with increased blood flow or decreased resistance index and greater than 10 cm or a growth rate greater than 3.5 cm per week were noted to have a higher risk for malignancy.
Hoover and Jenkins addressed the incidence and differential diagnosis, benign cystic teratoma (7% to 37%), serous cystadenoma (5% to 28%), mucinous cystadenoma (3% to 24%), endometrioma (0.8% to 27%), paraovarian cyst (<5%), and leiomyoma (91% to 25%). Ovarian malignancy accounted for 1% to 8%, most of which were low malignant potential (LMP).
Other risks related to adnexal mass in pregnancy include rupture and obstruction regarding labor. Randomized prospective data focused are lacking with regard to pregnant patients with adnexal masses addressed surgically versus expectant management. Complications that include preterm labor and spontaneous abortion must be included in the overall assessment and management. Evaluation of oncofetal antigens includes alpha-fetoprotein, human chorionic gonadotropin (hCG), lactate dehydrogenase, estradiol, and testosterone.
Before operative intervention, a complete assessment of the fetus—including ultrasound to rule out a lethal anomaly and to document cardiac activity—is in order. The optimal time for elective surgery is during the second trimester. The patient should be informed of the increased risk of preterm labor and delivery. The patient should be placed in the left lateral tilt position to avoid inferior vena cava compression and associated uteroplacental insufficiency. Postoperatively, the fetus should be placed on continuous fetal heart rate monitoring.
The most effective approach in the management of adnexal masses during pregnancy remains a point of controversy (i.e., laparoscopy vs. laparotomy). In a series of 88 pregnant women who underwent 93 surgical procedures for suspected adnexal pathology, laparoscopy was performed during the first trimester in 39 patients. The remaining 54 patients underwent laparotomy, 25 during the first trimester and 29 during the second trimester. Neither intraoperative nor postoperative internal complications were reported in the series. Five of thirty-nine women undergoing the first-trimester surgery had a spontaneous abortion. During the first trimester, a Veress needle was used for insufflation, and the procedure was in essence conducted in a manner virtually identical to that in the nonpregnant state (i.e., closed laparoscopy). It was concluded that laparoscopic gynecologic surgery is safe during pregnancy when conducted in the first trimester.
ULTRASOUND
Ultrasound is useful in predicting malignancy (
Table 28.2). Characteristic features of benign versus malignant neoplasms have been reported. Collated data from studies of ultrasound accuracy in the prediction of malignancy have an average positive predictive value of 74% and an average sensitivity of 88% (
Tables 28.3 and
28.4).
Weiner and coworkers have used transvaginal color flow imaging before exploratory surgery to study the impedance to blood flow in women with an adnexal mass. Intramural blood vessels consistently demonstrated low impedance to flow with a pulsatility index less than 1:16 in women with malignant tumors. The sensitivity and specificity of the preoperative pulsatility index in detecting malignant ovarian tumors were 94% and 97%, respectively. Kurjak and colleagues found that vessels with a low resistance index near the center of the mass or within papules or septa were highly correlated with malignancy. Therefore, transvaginal color flow imaging may be a useful clinical tool in the preoperative evaluation of ovarian masses.
Doppler resistance index has been used as a “vascular” scoring system. Color Doppler ultrasonography appears to be a reliable method in presurgically evaluating ovarian neoplasms.
Transvaginal color Doppler sonography has identified the following parameters as useful in determining malignant versus benign ovarian masses: number of vessels detected in each tumor, tumor vessel location (central vs. peripheral), peak systolic velocity, lowest resistance index, mean resistance index,
lower pulsatility index, and mean pulsatility index. Color Doppler signals were detected in 100% of malignant masses and 75% of benign masses, with the difference being statistically significant as reported by Alcazar and associates. Tumor vessel location appears to be central in virtually all malignant masses. Overall, the receiver operating characteristic curves generated can be used to predict malignant processes. The lowest resistance index was associated with the majority of malignant tumors.