Stillbirth and Intrauterine Fetal Demise
Robert M. Silver
Few obstetric complications are as emotionally devastating for families as antepartum stillbirth. Since the mid 20th century, there has been a tremendous decrease in the rate of stillbirth due to improved prevention and treatment of conditions such as diabetes, hypertension, and red cell alloimmunization. However, stillbirth rates have reached a plateau or have only modestly declined over the past several decades. This is in contrast to neonatal death rates, which continue to drop substantially. Currently, stillbirth after 20 weeks gestation affects over 1 in 200 pregnancies and is considerably more common than sudden infant death syndrome and intrapartum stillbirth in developed countries. Nonetheless, relatively more public attention and research have focused on infant rather than fetal mortality. Thus, fetal death remains a common, important, and poorly understood obstetric problem.
This chapter will review the nomenclature, epidemiology, causes, risk factors for, management of, and recurrence risk for stillbirth. Emphasis will be placed on clinically relevant issues, recent developments, and areas of controversy.
Terminology
The terminology of pregnancy loss can be confusing and often varies among and even within countries. Traditionally, abortions (or miscarriages) refer to pregnancy losses prior to 20 weeks gestation, while fetal deaths or stillbirths are used to describe losses after 20 weeks gestation. The threshold of 20 weeks is somewhat arbitrary, and it may be more useful to classify pregnancy losses by stages of gestational development. For example, pregnancy losses could be classified as pre-embryonic or anembryonic (conception to 5 weeks gestation), embryonic (6 to 9 weeks gestation), and fetal (after 10 weeks gestation) losses. In the past, anembryonic losses were termed blighted ova. This term is best avoided. Another approach would be to describe losses prior to 20 weeks gestation as early losses and those after 20 weeks gestation as late losses.
It is important to specify the timing in gestation and nature of pregnancy loss as accurately as possible. This can be difficult because death or failure of growth of the conceptus may precede clinical symptoms of miscarriage by days or weeks. Thus, ultrasound findings, histologic examination, and human chorionic gonadotropin (hCG) levels often are more useful than clinical data when characterizing pregnancy losses. The etiologies of pregnancy losses vary over gestation. Genetic problems are more common in pre-embryonic or anembryonic losses, while antiphospholipid syndrome (APS) and heritable thrombophilias are more likely in losses after 10 weeks gestation. Also, the recurrence risk of pregnancy loss is influenced by gestational age. Pregnancy losses tend to recur at similar times during gestation, and in patients with recurrent pregnancy loss, those with fetal deaths after 10 weeks gestation have worse subsequent pregnancy outcomes than women with recurrent early losses.
Stillbirth often is defined as the death of a fetus after 20 completed weeks of gestation. In cases of uncertain gestational age, losses weighing >500 g are considered
stillbirths. However, others believe that a more useful definition would include death of a fetus past the age of viability. This has prompted the use of 24 or even 28 weeks gestation as a threshold for defining stillbirth. Another controversial issue is whether or not to include both antepartum and intrapartum deaths as stillbirths. Some but not all studies distinguish between the two. This is an important distinction, as causes and strategies to reduce antepartum and intrapartum losses are quite different. Intentional fetal death (e.g., pregnancy termination in the setting of fetal anomalies or preterm premature rupture of membranes) poses another problem for vital statistics. Pregnancy terminations often are excluded when counting stillbirths, but the issue remains controversial. It seems appropriate to exclude terminations as a distinct entity. However, in many cases, these fetuses would be stillborn if the pregnancy were allowed to continue. As such, their exclusion might alter the true impact of stillbirth on a population.
stillbirths. However, others believe that a more useful definition would include death of a fetus past the age of viability. This has prompted the use of 24 or even 28 weeks gestation as a threshold for defining stillbirth. Another controversial issue is whether or not to include both antepartum and intrapartum deaths as stillbirths. Some but not all studies distinguish between the two. This is an important distinction, as causes and strategies to reduce antepartum and intrapartum losses are quite different. Intentional fetal death (e.g., pregnancy termination in the setting of fetal anomalies or preterm premature rupture of membranes) poses another problem for vital statistics. Pregnancy terminations often are excluded when counting stillbirths, but the issue remains controversial. It seems appropriate to exclude terminations as a distinct entity. However, in many cases, these fetuses would be stillborn if the pregnancy were allowed to continue. As such, their exclusion might alter the true impact of stillbirth on a population.
Epidemiology
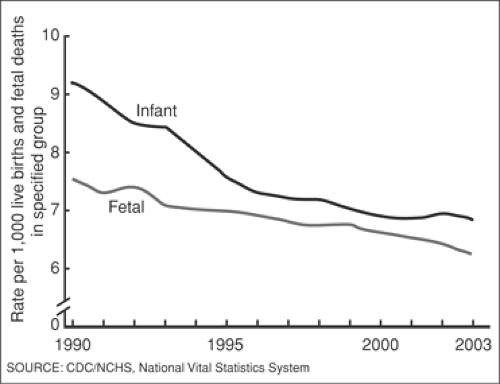
In 2003, the stillbirth rate (after 20 weeks gestation) in the United States was 6.23 per 1,000 live births. This represents a slight and steady decline since 1990, when the rate was over 7.5 per 1,000 live births (Fig. 23.1). Just over one half of stillbirths in the United States occur between 20 and 27 weeks gestation. It is important to note that this may underestimate the true rate, as stillbirths are likely underreported. The scope of the problem is considerably higher in many parts of the world. Stillbirth rates are estimated to range from 5 per 1,000 births in rich countries to 32 per 1,000 births in southern Asia and sub-Saharan Africa. The estimated number of global stillbirths per year is 3.2 million.
In the United States, stillbirth rates continue to be increased in non-Hispanic black women. In 2003, the fetal mortality rate for non-Hispanic blacks was 11.56 per 1,000 births compared with 4.94 per 1,000 births for non-Hispanic whites. Fetal mortality also is relatively higher for teenagers, unmarried women, and women over 35 years of age.
It is difficult to obtain an accurate estimate of the fetal death rate between 10 and 20 weeks gestation, because such data are not routinely collected. According to the National Survey of Family Growth, estimates of total fetal losses per year in the United States are about one million. The majority of these occur prior to 20 weeks gestation.
Classification
Numerous classification schemes have been used to catalog stillbirths based on etiology. This has proved difficult for several reasons. First, it often is difficult to be certain of a “cause” of death. Many risk factors such as diabetes, obesity, or heritable thrombophilias are associated with an increased risk of fetal death. However, the vast majority of women with these risk factors have live-born infants. Thus, it is difficult to be certain that these factors led to the stillbirth. Second, there may be more than one potential cause of stillbirth in the same patient. For example, what would be considered the etiology in a fetus with trisomy 18 that also has evidence of bacterial infection? Stillbirth may be due to the additive or interactive effects of several disorders. Finally, a cause of death often is never determined. In many cases, this is due to a lack of systematic evaluation into potential etiologies. However, a cause of death may not be found despite a comprehensive evaluation. This is especially true in cases of term stillbirth.
Because of these problems, no single classification scheme has been universally adopted, and new ones are being developed. Additional confusion arises from the use of different definitions for fetal death, the occasional inclusion of neonatal death, and different standards regarding causes versus associations in the classification schemes. The Wigglesworth classification probably is in most widespread use today (Table 23.1). A problem with all of the classification systems is that many stillbirths remain unexplained. Recently, Gardosi and colleagues introduced a new system that explains a much larger proportion of fetal deaths than prior schemes (Table 23.2). However, many of the etiologies in the new system may be risk factors such as small-for-gestational-age fetus (SGA) rather than etiologies. Forthcoming international conferences hopefully will facilitate the development of uniform definitions and classification systems for fetal death.
TABLE 23.1 Wigglesworth Classification | ||
|---|---|---|
|
Etiologies and Risk Factors
Fetal Conditions
Congenital Abnormalities
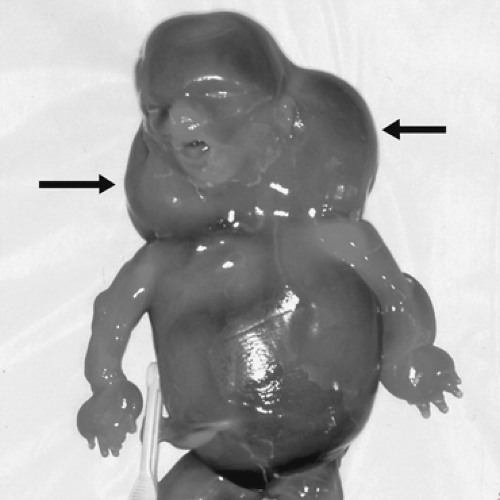
Genetic abnormalities and fetal malformations account for a significant proportion of stillbirths. Abnormal karyotypes are present in 6% to 12% of stillbirths after 20 weeks gestation. The rate of chromosomal abnormalities is considerably higher in first-trimester pregnancy losses and is probably intermediate in losses between 10 and 20 weeks gestation. This likely underestimates the true rate of chromosomal abnormalities, because karyotype is not always successfully obtained in cases of stillbirth. The odds of an abnormal karyotype are increased in the setting of fetal malformations, dysmorphic features, or SGA fetus. Conversely, the chances of an abnormal karyotyope is low (about 2%) in stillbirths greater than 20 weeks gestation with no apparent phenotypic abnormalities. The most common single abnormality in stillbirths is monosomy X (Fig. 23.2), occurring in 23% of cases with abnormal karyotype. Trisomies including 21, 18, and 13 also are common. This is in contrast to first-trimester losses, wherein trisomy 16 accounts for the majority of abnormalities.
Many fetuses have malformations or other congenital abnormalities without necessarily having chromosomal abnormalities. Up to 35% of stillborn infants undergoing perinatal autopsy have abnormalities including malformations, syndromes, and dysplasias. Approximately 25% of these will have an abnormal karyotype. However, it is likely that many of these fetuses have genetic abnormalities that are not identified by traditional karyotype.
Some fetuses may have microdeletions or additions that are too small to be identified by cytogenetics. Newer molecular genetic technology using a technique termed comparative genomic hybridization (CGH) may identify these smaller lesions. Similar abnormalities have been reported in cases of unexplained mental retardation with normal karyotype. Other cases of stillbirth are due to single gene mutations that require specific targeted assays for diagnosis. Many are autosomal recessive conditions such as glycogen storage diseases, hemoglobinopathies, and other metabolic disorders. There are probably several other single gene disorders responsible for some cases of stillbirth. Another potential genetic cause of pregnancy loss is confined placental
mosaicism. This refers to abnormal chromosomes in some or all of the placental tissue with normal fetal karyotype. In turn, this leads to abnormal placental function resulting in fetal growth impairment or death. Continued advancements in molecular genetic technology should enhance our ability to identify previously unrecognized genetic causes of stillbirth.
mosaicism. This refers to abnormal chromosomes in some or all of the placental tissue with normal fetal karyotype. In turn, this leads to abnormal placental function resulting in fetal growth impairment or death. Continued advancements in molecular genetic technology should enhance our ability to identify previously unrecognized genetic causes of stillbirth.
TABLE 23.2 Relevant Condition of Death (ReCoDe) Classification | ||
|---|---|---|
|
Infection
Infections are another biologically plausible, generally accepted cause of stillbirth, accounting for 10% to 25% of stillbirths in developed countries. The proportion of stillbirths due to infection (especially bacterial infection) is even higher in developing countries. It is important to be careful when attributing fetal death to infection. Mothers often have vaginal infection or colonization or systemic viral infections that may have nothing to do with a stillbirth. Fetal autopsy and placental histologic evaluation are extremely helpful in proving that infection truly is a cause of stillbirth. For example, positive cultures of group B streptococcus in fetal lungs is convincing evidence of causality, while positive maternal serology for cytomegalovirus (CMV) in a case with no evidence of infection on fetal autopsy is not.
Bacterial Infection
Bacterial infections implicated in stillbirth are most commonly due to ascending organisms from the genital tract. Bacteria such as Escherichia coli, Klebsiella, Ureaplasma urealyticum, Mycoplasma hominus, Bacterioides species, and group B streptococcus track from the vagina, through the cervix, and into the amniotic fluid, where they may be swallowed by the fetus, sometimes leading to infection. Less commonly, bacteria such as Listeria monocytogenes can be hematogenously transmitted to the fetus. It is noteworthy that some organisms usually cause clinically apparent intra-amniotic infection, while other indolent organisms such as L. monocytogenes may cause vague symptoms that are difficult to diagnose. Although rare, fetal death also may occur because of severe systemic maternal infection. This is thought to be due to the propagation of inflammatory mediators leading to uterine ischemia, hypoxia, and preterm labor.
Viral Infections
The most common viral infection that has been linked to fetal death is parvovirus B19 (Fig. 23.3). This virus is trophic for erythrocyte precursors and myocardial cells. Death is thought to be caused by fetal anemia, hydrops, and/or myocardial dysfunction. Parvovirus is most likely to cause fetal death after infection in the first two trimesters. Late fetal death due to the virus is rare. The organism has been reported in up to 15% of cases of fetal death when polymerase chain reaction (PCR) was used to detect parvovirus nucleic acid in the fetus or placenta. However, parvovirus has been found in <1% of fetal deaths in series that did not systematically assess for the virus. The true proportion of fetal deaths due to parvovirus is likely somewhere in between these figures. Viruses are difficult to culture, and PCR
probably identifies cases that would have been otherwise missed. Conversely, finding viral nucleic acid in the fetus or placenta without histologic evidence of viral infection may be represent a false-positive result.
probably identifies cases that would have been otherwise missed. Conversely, finding viral nucleic acid in the fetus or placenta without histologic evidence of viral infection may be represent a false-positive result.
CMV is the most common fetal/neonatal viral infection. Although cases have been reported, CMV rarely causes stillbirth. Coxsackie A and B virsuses are associated with stillbirth. They appear to cause death through placental inflammation, myocarditis, and hydrops. Numerous other viruses may sporadically cause stillbirth, but none accounts for more than a handful of cases.
Other Infections
Other types of infection also may cause stillbirth. The risk of stillbirth from syphilis increases with advancing gestation as the responsible spirochete, Treponema pallidum, may cross the placenta and directly infect the fetus. Syphilis is a major cause of fetal death in developing countries and persists in some parts of the United States. Infections such as malaria, syphilis, and toxoplasmosis account for an important number of stillbirths in endemic areas. As with viruses, numerous other spirochetes and other organisms cause sporadic stillbirth.
Small-for-Gestational-Age Fetus
SGA fetus is a major risk factor for fetal death. The risk for stillbirth increases with decreasing birth-weight percentile and likely occurs through several mechanisms, including an increased risk of genetic abnormalities, placental insufficiency, and other factors. It is important to recognize that SGA is a risk factor rather than a cause of fetal death. Indeed, the vast majority of SGA fetuses are constitutionally small (healthy fetuses in the lower 10% for weight). Also, intrauterine growth restriction (IUGR), rather than SGA, is associated with stillbirth. However, it often is difficult to determine whether a fetus is growth restricted. This implies a downward inflection in the rate of fetal growth, which requires serial measurements that are not available for many pregnancies. Second, population-based growth curves or percentiles for fetal weight do not account for an individual fetsus’s inherent growth potential. For example, a 6-pound infant may be growth restricted if its siblings all weighed 8 pounds at birth.
Nonetheless, SGA is a strong risk factor for fetal death. Also, the smaller the birth weight for gestational age, the higher the risk for fetal death. This dose-response curve provides robust evidence that SGA is a meaningful risk factor for stillbirth. The use of customized rather than population-based growth curves attempts to account for individual growth potential. A Swedish study using such growth curves noted an odds ratio for stillbirth of 6.1 (95% confidence interval [CI] 0.8 to 1.9) for SGA compared with average-for-gestational-age (AGA) fetuses. Umbilical artery Doppler velocimetry (or other antenatal surveillance) may help to distinguish between SGA fetuses that are constitutionally small and those at risk for stillbirth.
Maternal Conditions
Demographics
Race is one of the strongest risk factors for stillbirth. Non-Hispanic blacks have stillbirth rates that are over double that of non-Hispanic whites. The increase in the risk for fetal death persists, even when factors such as access to prenatal care are accounted for. In part, this may be due to relatively higher rates of medical and obstetric complications in blacks. However, other factors likely play a role as well. Approximately two thirds of the excess mortality in blacks occurs at 20 to 27 weeks gestation and one third at 28 weeks or more.
Advanced maternal age (>35 years of age) is associated with a progressive increase in the risk for stillbirth. In one population-based study, compared to women 20 to 34 years of age, the odds ratio for stillbirth in women 35 to 39 years of age was 1.28 (95% CI 1.24 to 1.32). It was 1.72 (95% CI 1.6 to 1.81) for those >40 years of age. Maternal age ≥35 years of age is an independent risk factor for stillbirth, even after adjusting for potential confounding variables such as genetic abnormalities or underlying medical conditions. This is of increasing concern, as a larger proportion of pregnancies are occurring in women ≥35 years of age.
Another risk factor for stillbirth that is increasing in frequency is obesity. Most studies have reported at least a twofold increase in the risk of fetal death in women with a body mass index (BMI) ≥30. A recent study of 25,000 women noted an odds ratio of 2.8 (95% CI 1.5 to 5.3) for stillbirth in women with BMIs ≥30 compared with a normal BMI of 18.5–24.9. Obese women have an increased risk for many other conditions such as diabetes and hypertension, which are known to be associated with stillbirth. However, after controlling for comorbidities, age, smoking, and other potential confounders, the adjusted odds ratio for stillbirth in obese women was 3.1 (95% CI 1.6 to 5.9).
There are several other maternal risk factors for stillbirth. Some but not all studies have reported an increased stillbirth risk in women with prior cesarean deliveries. Another risk factor is abnormal levels of maternal serum markers in cases with normal fetal karyotype. For example, low concentrations of pregnancy-associated plasma protein A in the first trimester and elevated maternal serum alpha-fetoprotein in the second trimester are associated with an increased risk of placental insufficiency and stillbirth. An excellent summary of available epidemiologic literature regarding risk factors for stillbirth is shown in Table 23.3.
Medical Disorders
Maternal medical diseases are estimated to contribute to 10% of fetal deaths. It is difficult to be certain as to whether these conditions are causes or risk factors for stillbirth, because most affected women do not have stillbirths. Also, medical and obstetric management can have a dramatic influence on pregnancy outcome in women with these
conditions. Examples include the tremendous decrease in perinatal mortality due to diabetes and hypertension over the past 50 years.
conditions. Examples include the tremendous decrease in perinatal mortality due to diabetes and hypertension over the past 50 years.
TABLE 23.3 Estimates of Maternal Risk Factors and Risk of Stillbirth | |||
|---|---|---|---|
|