Gynecologic Ultrasound
Mika Thomas
Bradley J. Van Voorhis
The use of sonography has become widespread in gynecology. Its accessibility, relatively low cost, and high patient acceptance make it applicable as an initial step in assessing many gynecologic disorders. This chapter discusses and illustrates the common uses of ultrasound (US) in the evaluation and treatment of a variety of gynecologic disorders. The subsections are divided according to the most common indications for gynecologic US, and a list of suggested readings is available at the end of the chapter for further in-depth reading on the subject.
Sonographic Instrumentation and Technique
US uses high frequency sound waves at various frequencies to allow imaging of internal organs and vessels. The waves emitted by the transducer pass through the soft tissue, and a portion of the wave reflects off perpendicular tissue/fluid interfaces. This reflection, or return echo (much like a sonar), is detected by the transducer, while the remaining portion continues through until the waves encounter another perpendicular tissue/fluid interface, sending back another reflected echo delayed in time. The US machine collates the information from the reflected echoes, based on the time required for their return and intensity, and reconstructs a two-dimensional (2D) sonographic image. Both the origin of the signal, the transducer, and the receiver are contained in the same unit. Three-dimensional ultrasonography (3D-US) characterizes an entire soft tissue volume by storing multiple 2D images, and the computer software rapidly collates the multiple 2D images thus yielding a 3D image. Once the information is electronically stored, this computerized storage system allows reconstruction of images within the defined volume in any plane and provides the opportunity to reorient the scan relative to internal soft tissue landmarks, which enhances the usefulness of the technology.
US is particularly useful in defining the internal acoustic characteristics of a soft tissue structure, thus distinguishing fluid-filled structures from solid structures. This makes US the imaging modality of choice in evaluating the ovary for cysts or neoplasms. This imaging technique is not as well adapted to distinguish solid structures from other adjacent, or surrounding, solid structures. A perfect example of this is the ease at which an intrauterine gestational sac can be visualized, while a comparably sized intracavitary uterine polyp or fibroid may be quite difficult to distinguish from the adjacent endometrium or myometrium with a similar acoustic appearance.
Diagnostic US of the pelvic organs can be performed by using the transabdominal sonography (TAS) approach, in which the uterus and adnexa are imaged through a distended urinary bladder, or transvaginal ultrasound (TVUS), where the probe is inserted into the vagina for imaging with an empty bladder. In general, the lower the frequency emitted by the US transducer, the further the penetration and the deeper the window of visibility but the smaller the amount of discrimination between soft tissues. Therefore, TAS uses lower frequency sound waves (3.5 to 5.0 MHz) to allow for the deeper penetration required to visualize intra-abdominal structures beneath the subcutaneous tissue, which can be quite variable in depth between patients. A TAS is most useful in fully assessing large masses that extend out of the pelvis or in situations where TVUS cannot be performed, such as in pediatric or adolescent patients. Due to problems visualizing the pelvic organs through intervening tissues such as the small and
large bowel or the anterior abdominal wall, the transabdominal approach is best performed with a fully distended urinary bladder, enabling a better acoustic window without interfaces to reflect echoes to visualize the uterus and adnexa. However, TAS remains limited by body habitus and any intervening bowel or preperitoneal fat, which can increase artifacts and scatter the incident US beam.
large bowel or the anterior abdominal wall, the transabdominal approach is best performed with a fully distended urinary bladder, enabling a better acoustic window without interfaces to reflect echoes to visualize the uterus and adnexa. However, TAS remains limited by body habitus and any intervening bowel or preperitoneal fat, which can increase artifacts and scatter the incident US beam.
TVUS uses higher frequency sound waves (5 to 8 MHz), which allow higher image resolution but with less tissue penetration, thus limiting the ability to “see” objects more distant than 10 cm clearly but images the closer tissues much better. TVUS is a better modality with which to image obese women because the US transducer can be positioned immediately adjacent to the uterus and ovaries without the hindrance of subcutaneous tissue. For most applications, a slightly curved transducer probe that has high line density affords the most detailed image (Fig. 30.1). It becomes increasingly difficult to see the uterine fundal region if it extends above the pelvis, as in the case of a large fibroid uterus or the pregnant uterus in the second and third trimesters. By contrast, the transvaginal component of an examination of the uterus typically can better visualize the proximity of fibroids to the endometrial cavity than can TAS. Image clarity with TVUS usually is superior when evaluating ovarian abnormalities as compared with TAS and also can be helpful as a means of distinguishing between ovarian and uterine masses. In addition, TVUS with Doppler sonography affords assessment of the flow of blood within vessels adjacent to and within the uterus and ovaries.
TVUS is best performed in patients placed in the lithotomy position with an empty bladder. The vaginal probe should be disinfected and covered with a sheath prior to insertion. For optimal patient comfort, the probe should be inserted into the vagina while the operator’s finger gently depresses the posterior introitus. Alternatively, the patient may insert the probe herself. A US examination must be thorough and reproducible; for this reason, most would suggest performing each US in a proscribed fashion, following the same routine with every scan. A complete pelvic US examination begins with images of the uterus as the central pelvic landmark both in the sagittal and coronal axes. The probe can be withdrawn into the midvagina and directed anteriorly to provide views of an anteflexed uterus. The retroflexed uterus is imaged easily without major manipulation of the probe because it is closer to the plane of the vagina, though some vaginal probes angle the US beam superiorly, making this somewhat more difficult or uncomfortable for the patient. The operator can then orient the probe for imaging the adnexal regions by using the internal iliac artery and vein as landmarks for delineation of the ovarian fossa. The normal ovary usually can be found just medial to the internal iliac artery and vein, but there is substantial variation in the location of the ovaries, particularly in women who have undergone pelvic surgery or
hysterectomy or who have enlarged ovaries that may extend upward out of the pelvis. Typically, enlarged ovaries or ovaries containing cysts are relatively easy to identify, while the normal postmenopausal ovary might be more difficult to visualize. The operator can use one hand to mildly compress the abdominal wall and evaluate the mobility of these organs. If there are no adhesions, the uterus and ovaries should move smoothly away from each other as the probe is advanced. This has been termed the sliding organ sign and, if absent, may suggest the presence of agglutinating pelvic adhesions.
hysterectomy or who have enlarged ovaries that may extend upward out of the pelvis. Typically, enlarged ovaries or ovaries containing cysts are relatively easy to identify, while the normal postmenopausal ovary might be more difficult to visualize. The operator can use one hand to mildly compress the abdominal wall and evaluate the mobility of these organs. If there are no adhesions, the uterus and ovaries should move smoothly away from each other as the probe is advanced. This has been termed the sliding organ sign and, if absent, may suggest the presence of agglutinating pelvic adhesions.
To evaluate the vascularity of an organ, US machines make use of the physics principle known as the Doppler effect. This principle states that sound or light waves reflected by a moving object will undergo a change in frequency proportional to the relative velocity of that object, toward or away from the transducer (e.g., like the difference in sound made by an approaching and departing train). Detecting these frequency changes can help to determine the blood flow to a structure being imaged. Transvaginal color Doppler sonography (TV-CDS) combines the anatomic information provided by TVUS with blood flow information provided by CDS (Fig. 30.2). TV-CDS assesses blood flow and resistance to blood flow in larger vessels supplying pelvic organs. The flow within a vessel can be characterized in several different ways by TV-CDS. First, the flow waveform recorded by the Doppler can be described (e.g., the presence or absence of diastolic flow through the vessel). Alternatively, the waveform can be analyzed by measuring resistive indices that indicate the downstream impedance to blood flow within a vessel. These resistive indices are only indirect measures of the actual blood flow to an organ. Commonly measured indices are the resistive index (peak systolic velocity minus minimum end diastolic velocity divided by peak systolic velocity) or the pulsatility index (peak systolic velocity minus end diastolic velocity divided by the mean velocity over the cardiac cycle.) These parameters are unitless values and are measures of relative impedance to forward flow. One must obtain signals from between 30 and 60 degrees to the longitudinal axis of the vessel in order to provide optimal waveforms. Actual blood flow volume and velocity can be determined in larger vessels, but these measurements are not accurate in the smaller vessels. Standard, frequency-based color Doppler is assigned red colors for flow toward the transducer and blue colors for flow away from the transducer.
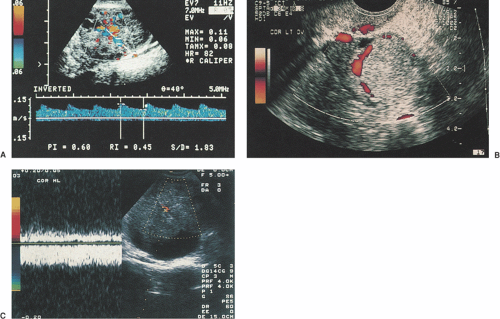 Figure 30.2 Transvaginal color Doppler sonography. A: TV-CDS showing low-impedance flow (resistive index = 0.45; pulsatile index = 0.60) within the wall of a corpus luteum. B: Amplitude color Doppler image of hemorrhagic corpus luteum showing no flow within the center of the mass, which contained an organized clot. C: Complex mass with low-impedance arterial and increased venous flow within an irregular solid area, which is highly indicative of ovarian cancer. (See Color Plate) |
A new type of processing known as power Doppler has been developed for the detection of flow within much smaller vessels. This technique is more sensitive, which allows for the detection of areas of low blood flow, and the information is independent of the angle of insonation of the vessel. The main disadvantages are that there is no information about speed or direction of flow, and there is high motion sensitivity leading to false readings. The principal
aim of power Doppler is to determine the simple presence or absence of flow, although computer analysis of images allows for quantification of blood flow for research purposes.
aim of power Doppler is to determine the simple presence or absence of flow, although computer analysis of images allows for quantification of blood flow for research purposes.
Clearly, the accuracy of TVUS is operator dependent, and significant clinical experience with anatomic correlation is required for effective use. For those who do not routinely perform pelvic sonograms as part of their practice but rather order them, it is crucial that they have a working understanding of not only the lexicon of US but also at least a rudimentary ability to interpret the images that are collected. US images and reports must also be saved for medicolegal reasons. US, to some extent, is subjective and documentation of results is incomplete without a narrative description of the overall impression by the operator.
While becoming more widely available and used in obstetric practice, the clinical value of 3D-US in gynecology over the standard 2D technique has yet to be demonstrated. With 3D-US, data is captured and stored during a “sweep” of the pelvis with the vaginal probe. This allows for the rapid acquisition of data and the ability to subsequently display the images in three dimensions. Since data from the entire volume of the pelvis has been stored, any 2D plane can be recreated that is oriented toward the internal organs and thus display the optimal section through the region of interest. For example, the true coronal image of the uterus can be displayed, which cannot be viewed in standard 2D images from an external orientation. 3D-US has been shown to be efficient and accurate in characterizing Müllerian abnormalities and in measuring ovarian and fibroid volumes and shows promise in some urogynecologic applications. Whether or not the improved imagery actually improves the detection rate of pathology has not been demonstrated for most gynecologic applications. An even more recent advance is the ability to display 3D images live on the screen (sometimes called live 3D- or “4D”-US). The true clinical advantage of these newer techniques currently is under investigation.
Abnormal Uterine Bleeding
Irregular uterine bleeding is a common presenting complaint for the gynecologist, and it is necessary to differentiate abnormal uterine bleeding (AUB) from dysfunctional uterine bleeding (DUB), as their management approaches differ greatly. DUB, which may only be diagnosed once organic or anatomic sources have been ruled out, is most commonly caused by anovulation due to estrogenized anovulation, or polycystic ovary syndrome (PCOS). Ultrasonic diagnostic criteria for PCOS is discussed later in the Infertility section. AUB, on the other hand, may be caused by a variety of conditions including uterine fibroids, endometrial polyps, endometrial hyperplasia or carcinoma, and endometritis. TVUS plays an important role in evaluating these women.
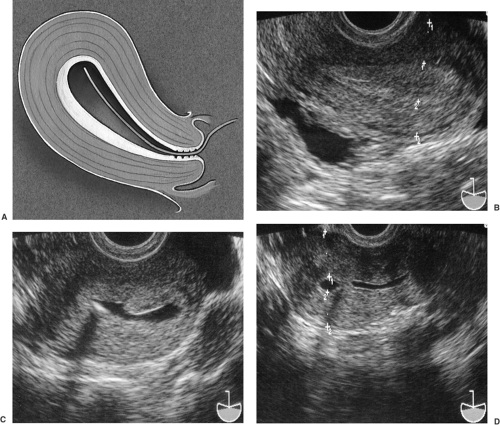
Because of the proximity of the transvaginal probe to the uterus, the endometrial and myometrial architecture can be accurately depicted in detail in most patients. One of the most important structures to image in the evaluation of a woman with AUB is the endometrial stripe. It becomes very clear when measuring the endometrial stripe that proper imaging technique is critical, as the endometrium is not a precise geometric shape and operator error can account for over- or underestimation of its thickness. Thus, it is of utmost importance to properly orient the scan of the endometrium in its greatest long-axis plane and maximal thickness in the fundal region, which optimizes its bilayer measurement (Fig. 30.3).
Normal endometrial thickness varies depending on both the patient’s reproductive endocrine status and the use of hormones. In women of childbearing age, the endometrial thickness changes according to the stage of the menstrual cycle. During menses, the normal endometrium is 3- to 5-mm thick with a mildly echogenic texture. As the endometrium proliferates in the periovulatory period, a multilayered texture can be seen with thicknesses ranging from 5 to 8 mm. The outer echogenic layer represents the basalis, whereas the inner layer is the enlarging functionalis. In the secretory phase, the endometrium becomes diffusely echogenic and enlarges up to 12 to 14 mm in thickness. This is in contrast to the thin endometrial stripe of the postmenopausal patient, which typically measures <3 mm unless the patient is taking hormonal therapy, which will lead to a thicker proliferative endometrium, or has true endometrial pathology.
Clinically, it is important to remember that the prevalence of different causes of AUB differ depending on the age of the woman. In general, premenopausal bleeding often is associated with pathology such as intracavitary fibroids or endometrial polyps and less commonly with endometrial neoplasia. In the postmenopausal patient, however, pathologies including atrophic endometritis, hyperplasia, and endometrial carcinoma are more common.
Indeed, one of the more valuable roles of TVUS is evaluating unexplained bleeding in the postmenopausal woman. A thickened or highly echogenic endometrium in a postmenopausal patient can suggest the presence of polyps, abnormal endometrial histology such as adenomatous hyperplasia, or even cancer. However, there is no endometrial stripe thickness above which carcinoma or hyperplasia is always found, and there is also no thickness below which cancer is never encountered. Many studies have been published assessing whether there is a stripe thickness cutoff below which one can safely presume a low likelihood of pathology and avoid invasive diagnostic procedures to assess endometrial histology. Meta-analyses have concluded that using 5 mm as the cutoff, irrespective of whether the patient is on hormone replacement therapy, results in a sensitivity of 92% in identifying any endometrial pathology and 96% for detecting carcinoma. An endometrial thickness of <5 mm was associated with a significantly reduced
risk of an underlying carcinoma. As suggested by these findings, a thin endometrial stripe reduces but does not eliminate the chance that there is an area of cancer present. Therefore, particularly in the face of continued bleeding or in a high-risk patient, tissue sampling should always be considered.
risk of an underlying carcinoma. As suggested by these findings, a thin endometrial stripe reduces but does not eliminate the chance that there is an area of cancer present. Therefore, particularly in the face of continued bleeding or in a high-risk patient, tissue sampling should always be considered.
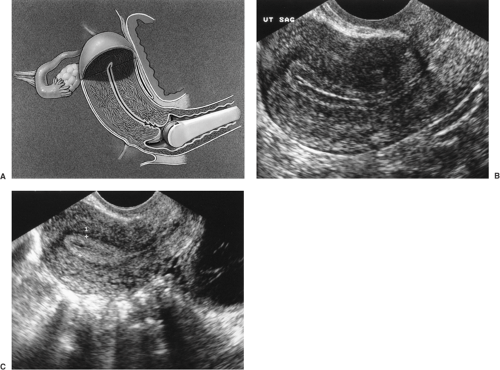 Figure 30.3 Endometrial disorders. A: Diagram showing proximity of the endometrium of an anteflexed uterus to the transvaginal probe. The field of view depicting the long axis of the uterus and endometrium is shown. (Drawing by Paul Gross, MS.) B: TVUS showing multilayered endometrium typical of follicular phase endometrium. C: Typical secretory phase endometrium demonstrating increased thickness (between cursors) and homogeneous echogenicity. (See Color Plate) |
In the premenopausal patient, there are several US findings that are suggestive of an intracavitary abnormality that might be the cause of abnormal bleeding such as a thickened endometrium or an endometrial stripe that is distorted or has an acoustic appearance that is heterogenous. Uterine fibroids, a common source of AUB, can result in any one of the mentioned US findings. Uterine fibroids typically are hypoechoic or of mixed echogenicity when compared with normal myometrium with “shadowing” or low ultrasonic reflection beyond the fibroid. Some fibroids contain calcifications that appear as areas of high acoustic reflection similar to that seen in bone. The endometrial stripe adjacent to the fibroid should be visualized to assess the fibroid’s effect on the endometrium. Submucosal fibroids typically displace and thin the overlying endometrium and sometimes are best visualized with saline infusion sonohysterography (SIS). Occasionally, fibroids may be present as solid masses in the adnexal regions, representing either interligamentary or pedunculated tumors.
Another common cause of AUB is the presence of endometrial polyps. These usually arise from the endometrium in the uterine fundus and appear as focal areas of increased endometrial thickening and/or irregularity. They may be pedunculated or sessile, and may be difficult to distinguish from intracavitary fibroids. Endometritis, also a cause of AUB, can produce a thickened endometrium, as well as accumulation of intraluminal fluid.
Saline infusion sonohysterography (SIS) has become an important and effective adjunct to standard TVUS. The endometrial cavity is a potential space where the anterior and posterior endometrium are in direct apposition to one another. Fibroids, polyps, and even scar tissue (intrauterine synechiae), such as that seen in Asherman’s syndrome, within the endometrial cavity are often difficult to visualize on standard TVUS because the US characteristics are similar to the contacting endometrium. Using saline infusion as an acoustic contrast medium to distend the endometrial cavity, enables visualization of structures within
the endometrial potential space in greater detail than with TVUS alone (Fig. 30.4). With saline infused into the cavity, the walls of the uterus separate, and polyps, intracavitary fibroids, and synechiae that might otherwise have been missed are readily visualized.
the endometrial potential space in greater detail than with TVUS alone (Fig. 30.4). With saline infused into the cavity, the walls of the uterus separate, and polyps, intracavitary fibroids, and synechiae that might otherwise have been missed are readily visualized.