(1)
National Institute of Pediatrics, Department of Dermatology, Insurgentes Sur 3700, México, D.F. 04530, USA
Abstract
Infections in immunocompromised children pose a challenge to dermatologists, both because the clinical presentation of common infectious diseases can be greatly modified making diagnosis difficult and because some of these infections have the potential to disseminate and have severe consequences, sometimes fatal. In many cases the skin is the easiest and most accessible way to make a correct diagnosis and initiate adequate treatment. This chapter describes some of the most important bacterial, fungal, viral, mycobacterial, and parasitic infections that children with altered immune systems present.
Keywords
ImmunodeficiencyImmunocompromisedInfectionsOpportunistic infectionsBackground/Introduction
Managing patients with alterations in the immune system poses a great challenge to dermatologists, since the clinical presentation and severity of even common infections are greatly modified by the underlying condition.
Epidemiology
Common pathogens in immunocompromised patients can be widespread and/or have the potential for serious consequences.
Opportunistic pathogens are more common in immunocompromised hosts.
Depending on the underlying immune alteration, some pathogens will be more common than others.
Children whose immune system is altered, either temporarily by medications or diseases, or chronically due to genetic or acquired conditions, are susceptible to many diverse types of infections that frequently have dermatologic manifestations. These can be infections caused by common pathogens that frequently affect immunocompetent hosts, but in immunocompromised patients they can be widespread and/or have the potential for serious consequences (e.g., cellulitis or furunculosis). They can also be caused by uncommon pathogens that are opportunistic and are particular of immunocompromised hosts (e.g., aspergillosis, zygomycosis). Finally, skin infections can be primary to the skin or secondary by dissemination from an internal non-cutaneous focus (e.g., fusariosis).
Clinical Presentation
Children with primarily cell-mediated immunodeficiency are at risk for invasive infections by fungal (Candida, Cryptococcus, Blastomyces, Histoplasma, or Coccidioides species), bacteria ( Nocardia, Salmonella species), mycobacteria, and viruses (herpes simplex virus, varicella zoster virus, cytomegalovirus, and Epstein-Barr virus [1].
Patients with myeloproliferative disorders or who have genetic conditions with profound neutropenia are more susceptible to bacterial ( Pseudomonas aeruginosa, enterobacteriaceae, Staphylococcus sp., Clostridium ) and fungal ( Aspergillus sp., Candida sp., Fusarium sp., Mucorales sp.) infections [1].
Patients with alterations in humoral immunity present with bacteria ( S. pneumonia, N. meningitidis, H. influenza ), encapsulated gram-negative bacilli, enteroviruses, and parasites like G. lamblia [1].
Depending on the underlying immune alteration, some pathogens are more common than others. Children whose immunodeficiency is primarily cell mediated, such as patients with Wiskott–Aldrich syndrome, Human Immunodeficiency Virus-Acquired Immunodeficiency Syndrome (HIV-AIDS), organ transplants, and certain types of cancers are at risk for invasive infections by fungi (Candida, Cryptococcus, Blastomyces, Histoplasma, or Coccidioides species), bacteria (Nocardia, Salmonella species), mycobacteria and viruses (herpes simplex virus (HSV), varicella zoster virus (VZV), cytomegalovirus (CMV), and Epstein-Barr virus (EBV) [1].
Patients with myeloproliferative disorders, who are undergoing chemotherapy or who have genetic conditions which frequently go through periods of profound neutropenia., such as Chediak–Higashi syndrome, Job syndrome, and chronic granulomatous disease, are more susceptible to bacterial (Pseudomonas aeruginosa, enterobacteriaceae, Staphylococcus sp., Clostridium) and fungal (Aspergillus sp., Candida sp., Fusarium sp., Mucorales sp.) infections [1].
On the other hand, children who have alterations in their humoral immunity due to asplenia, nephrotic syndrome, paraproteinemias, or primary hypogammaglobulinemias present with bacterial (S. pneumonia, N. meningitidis, H. influenza, and encapsulated gram-negative bacilli), enteroviruses, and parasites like G. lamblia [1].
Very frequently cutaneous lesions are accompanied by fever and systemic illness, making prompt diagnosis and treatment mandatory.
Pathogenesis
For all infections included in this chapter, there is a common pathogenesis. When the skin barrier is compromised, pathogens can enter and cause localized infection that can then disseminate or become systemic when the host’s immune system is not functioning properly. In the same way, infection can disseminate from internal organs such as lungs or gastrointestinal tract to the skin because of the altered immune system that cannot contain it [2].
For didactics in this chapter, we will categorize infections in immunocompromised children by the type of causative agent: bacterial, viral, mycobacterial, fungal, or parasitic. In each category we include epidemiology and demographics, clinical presentation, treatment, prognosis, and ongoing research.
Bacterial Infections
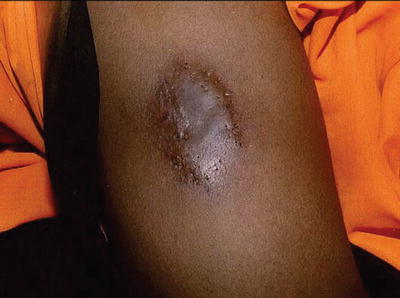
Immunocompromised patients can present infections by common skin pathogens such as Staphylococcus sp. and Streptococcus sp., but they can be more widespread or severe (Fig. 21.1). There are certain bacteria, however, characteristic of immunocompromised patients that have particular clinical manifestations.
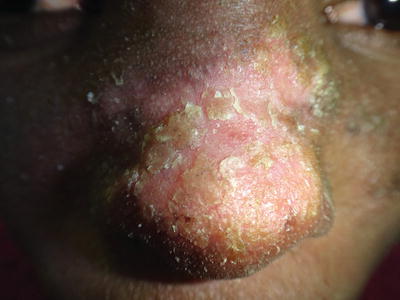

Fig. 21.1
Patient with Job syndrome or Hyper IgE syndrome and extensive impetigo by S. aureus
Pseudomonas aeruginosa is a very common cause of bacteremia in neutropenic patients with cancer (5–12 %), particularly acute leukemia. Up to 30 % of the patients with pseudomonal sepsis will have skin lesions [2]. Clinical manifestations include folliculitis, pustules, cellulitis, subcutaneous nodules, and the characteristic ecthyma gangrenosum. This entity presents as erythematous plaques or nodules that progress to become hemorrhagic bullae or necrotic ulcers, is usually associated with fever and general malaise (Fig. 21.2) [3]. Mortality is as high as 27 %, especially when lesions are around the perineal area [4].
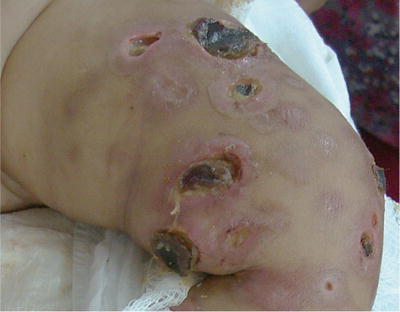

Fig. 21.2
Ecthyma gangrenosum by P. aeruginosa in a patient with HIV-AIDS. (Courtesy Prof. R Ruiz-Maldonado)
Other agents that can cause ecthyma gangrenosum-like lesions are S. maltophilia, Vibrio vulnificus, Escherichia coli, S. marcescens, S. aureus, Candida sp., Aspergillus sp., and Fusarium sp. [1, 4]. Diagnosis is usually made clinically, and the agent may be identified through tissue culture or blood cultures. Histologically, necrosis and vasculitis with minimal inflammation are characteristic. Treatment includes debridement, cytokine therapy with agents like granulocyte-colony stimulating factor (G-CSF), and intravenous antimicrobial treatment based on susceptibility patterns from culture [2].
Clostridium sp. like C. perfringens or C. septicum can cause bacteremia in patients with leukemia and malignancies. Skin lesions classically include rapidly spreading cellulitis or spontaneous gangrene. Prompt diagnosis is essential; patients develop sepsis and mortality is as high as 90 %. Treatment with surgical debridement and antibiotic therapy with penicillin with clindamycin or metronidazole should be initiated promptly.
Nocardia species are gram-positive, acid-fast bacteria that are common causes of infection in hosts with altered immune status. The most common species causing human infection are N. asteroides, N. braziliensis, and N. otitidiscaviarum. Breaks in the skin can result in cellulitis, abscesses, or subcutaneous nodules, often in a sporotrichoid pattern. There can be hematogenous spread to other organs as well. Histologically, branching filaments can be visualized with acid-fast stains, and tissue or blood culture to identify the species should be done. Treatment with sulfonamides combined with another agent according to susceptibility should be initiated and maintained for several weeks after clinical resolution [2].
Viral Infections
HSV infection is common when there is cell-mediated immune dysfunction; both reactivation and primary infection. Immunocompromised hosts present mucocutaneous vesicular or ulcerated lesions that are more chronic, extensive, and severe than in healthy hosts. A Tzanck smear from lesion exudate should be done to identify the typical HSV associated changes, and isolation of the virus in culture is diagnostic. Acyclovir is the mainstay of prevention and therapy; however resistance has increased in the past years [2].
VZV infections are frequent as well, resulting in more widespread and severe presentations of the typical vesiculopustular crusted lesions. These can be necrotic, hemorrhagic, and more painful in immunocompromised patients. Dissemination of the disease to lungs, central nervous system, and liver is also a potential complication to avoid. Treatment with high dose acyclovir should be started promptly. Prophylactic VZV immunoglobulin in these patients is also an option [2].
Cytomegalovirus (CMV) is a common opportunistic pathogen, particularly in post-transplant patients. Infection can be primary through donor organs, blood transfusions, or reactivation. Patients usually present with pneumonitis, hepatitis, encephalitis, retinitis, and bone marrow suppression. Skin lesions are nonspecific. The most common is an erythematous maculopapular eruption that should generate clinical suspicion so the pathogen can be sought for and identified through cultures. Treatment with intravenous ganciclovir is the first-line choice [2, 5].
Human papillomavirus infection (HPV) is a very common infection in all patients, but in patients with cellular immune defects there is persistence of lesions due to decreased clearance of the virus [6].
Epidermodysplasia verruciformis is a rare genodermatosis where there is vulnerability to HPV infection, particularly types 5 and 8. Children with HIV-AIDS infection can have severe HPV infection manifesting as widespread flat warts that are recalcitrant to treatment (Fig. 21.3). It has recently been found that these children have a form of acquired epidermodysplasia verruciformis, which can also be found in children with lymphoproliferative disorders, or organ transplants, where up to 53.8 % have warts that are more chronic and recurrent than in healthy hosts [7–10].
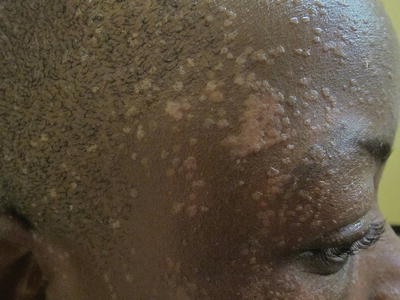

Fig. 21.3
Extensive HPV infection in a patient with HIV-AIDS
All children who are immunocompromised and have persistent, chronic HPV lesions should have typification of the virus causing the lesions done in order to identify high-risk oncogenic subtypes and treat them more aggressively as well as have strict follow-up.
Treatment options for cases recalcitrant to usual therapy with cryotherapy, salicylic acid, podophyllin, or cantharidin include imiquimod, squaric acid [11], and topical cidofovir [12, 13] with the advantage of decreasing the risk of residual hypo or hyperpigmentation associated to other treatments.
Mollusca contagiosa caused by a poxvirus is common, and often numerous and severe in immunocompromised children. It can be found in up to 6.9 % of transplanted children; often they are multiple and resistant to treatment [7]. In children with lymphoproliferative diseases atypical lesions can be present, such as giant or ulcerated mollusca [14]. Besides the usual physical or chemical treatments, a new option for treatment of recalcitrant cases in immunocompromised children is topical cidofovir [15, 16].
Mycobacterial Infections
Mycobacterium tuberculosis may produce cutaneous lesions after inoculation from an exogenous source, or dissemination from an endogenous source or hematogenous source. Immunocompromised children with severe malnutrition, malignancies, and HIV-AIDS are most at risk, especially Hispanic and non-Hispanic black children who have higher prevalence of positive tuberculin skin tests and tuberculosis (TB) [17, 18]. Pediatric patients with HIV infection frequently are coinfected with TB (up to 20-fold greater risk) and patients are more likely to be smear negative and nonreactive to skin testing, so a high index of suspicion should be maintained [19, 20]. Skin lesions can vary from purpuric papules to verrucous plaques or abscesses with draining sinuses (Fig. 21.4). Tissue examination for acid-fast bacilli, tissue culture, and histopathology should be done. Treatment with full regimen antituberculous therapy is needed: a 2-month intensive phase of daily isoniazid, rifampicin, pyrazinamide, and ethambutol or streptomycin followed by a 4-month continuation phase with isoniazid and rifampicin [21, 22].
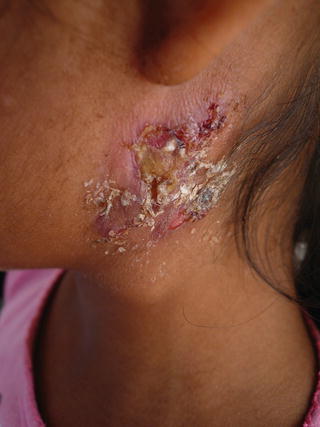

Fig. 21.4
M. tuberculosis infection extending from cervical lymph nodes in a severely malnourished girl
Systemic bacillus Calmette-Guérin infection (BCGitis) is characterized by multiple and disseminated papulonodular or ulcerated cutaneous lesions associated with systemic involvement after application of BCG vaccine. It presents in patients with severe cellular-inherited immune deficiencies, such as severe combined immunodeficiency or chronic granulomatous disease, and children with HIV-AIDS. The estimated prevalence is between 0.1 and 4.3 per million vaccinated. The treatment includes full antituberculous regimen and interferon-gamma if needed, abscess drainage, and/or local isoniazid injection [17, 23].
Non-tuberculous mycobacteria (NTM) are extremely common causes of infection in immunocompromised children, and very frequently there is no history of skin trauma (contrary to mycobacterial infections in healthy hosts). There are more than 100 species of NTM, and some of the most common pathogens in immunocompromised hosts are M. fortuitum, M. chelonae, M. avium-intracellulare, M. haemophilum, and M. kansasii. Patients with specific immunodeficiencies, such as interferon-gamma receptor, can present recurrent infections by different species [24]. There is a wide array of cutaneous manifestations associated with these pathogens: papules, vesicles, nodules, ulcers, draining sinuses, and cellulitis-like lesions (Fig. 21.5). Biopsy specimens exhibit granulomatous and suppurative inflammation, and acid-fast bacilli may be observed. The gold standard for diagnosis is tissue culture to identify the causative species and antibiotic susceptibility. Treatment should include at least two antibiotics for at least 3 months [25].